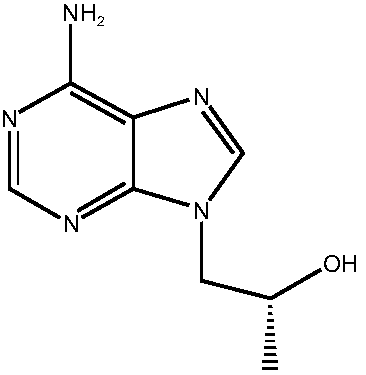
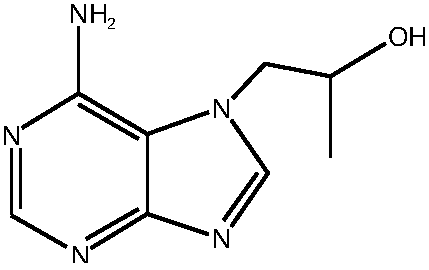
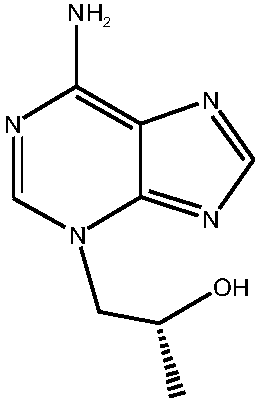
Preparation method of high-purity tenofovir
A tenofovir, high-purity technology, applied in the field of chemical drug synthesis, can solve the problem that the product purity is only 99.3%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention provides a kind of preparation method of high-purity tenofovir, at least comprises the following steps:
[0034] (1) Add R-1,2-propanediol, diethyl carbonate and sodium ethylate to the reactor, heat, keep warm and react, and the reaction is terminated. Unreacted diethyl carbonate is steamed out by distillation under reduced pressure, cooled to room temperature, The insoluble matter was filtered off, and concentrated to dryness under reduced pressure to obtain R-propylene carbonate;
[0035] (2) add toluene, catalyzer in reactor, carry out rectifying column rectification 6-8h, after rectifying completes, decompress and concentrate dry, obtain the catalyst with high purity;
[0036] (3) Mix adenine and R-propylene carbonate in the first solvent under the protection of nitrogen, under alkali catalysis, heat, heat preservation reaction, reaction termination, obtain 9-(2-hydroxypropyl) adenine crude product, filter , concentrating under reduced pressur...
Embodiment 1
[0088] The preparation method of high-purity tenofovir at least comprises the following steps:
[0089] (1) Add R-1,2-propanediol, diethyl carbonate and sodium ethoxide to the reactor, heat to 105-108°C, keep warm for 8 hours, the reaction is terminated, and the unreacted diethyl carbonate is distilled under reduced pressure. out, cooled to room temperature, filtered off insoluble matter, concentrated to dryness under reduced pressure to obtain R-propylene carbonate; the molar ratio of the R-1,2-propanediol to the diethyl carbonate and the sodium ethoxide was 1:1.15 : 0.82;
[0090] (2) add toluene, catalyzer in reactor, carry out rectifying column rectification 6-8h, after rectifying completes, decompression concentrates dry, obtains the catalyst that purity is high; The weight ratio of described toluene and described catalyzer is 11 : 1; the catalyst is potassium tert-butoxide;
[0091] (3) Mix adenine and R-propylene carbonate in the first solvent under the protection of ni...
Embodiment 2
[0096] The preparation method of high-purity tenofovir at least comprises the following steps:
[0097] (1) Add R-1,2-propanediol, diethyl carbonate and sodium ethoxide to the reactor, heat to 105-108°C, keep warm for 8 hours, the reaction is terminated, and the unreacted diethyl carbonate is distilled under reduced pressure. out, cooled to room temperature, filtered off insoluble matter, concentrated to dryness under reduced pressure to obtain R-propylene carbonate; the molar ratio of the R-1,2-propanediol to the diethyl carbonate and the sodium ethoxide was 1:1.15 : 0.82;
[0098] (2) add toluene, catalyzer in reactor, carry out rectifying column rectification 6-8h, after rectifying completes, decompression concentrates dry, obtains the catalyst that purity is high; The weight ratio of described toluene and described catalyzer is 11 : 1; the catalyst is potassium tert-butoxide;
[0099] (3) Mix adenine and R-propylene carbonate in the first solvent under the protection of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com