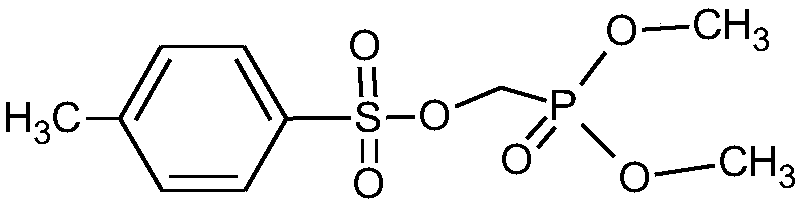
Method for synthesizing diethyl (tosyloxy)methylphosphonate
A technology of diethyl toluenesulfonyloxymethylphosphonate and diethyl phosphite is applied in the field of synthesizing diethyl p-toluenesulfonyloxymethylphosphonate, which can solve the problems of unsuitability for industrialization, troublesome post-processing and environmental protection High pressure and other problems, to achieve the effect of simple post-processing, short reaction time, and low environmental protection pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
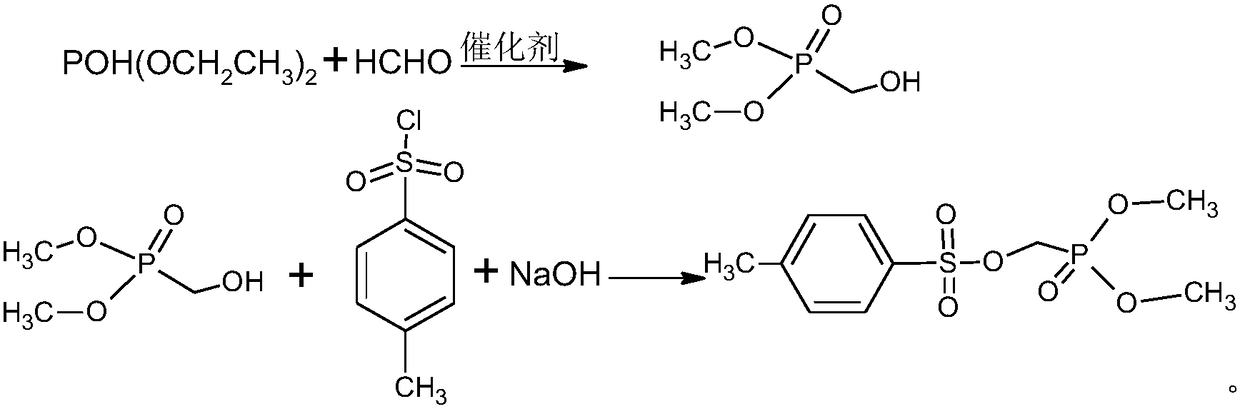
[0034] (1) In a 500ml three-neck flask, add 50g of 37% formaldehyde solution, 3.0g of sodium carbonate, cool down to 0°C, add 60g of diethyl phosphite dropwise, and keep the temperature between 0-5°C during the dropping process , The addition was completed in 2 hours, and after the addition was completed, the reaction solution was obtained by incubating at 2°C for 2 hours. Sampling detection, diethyl phosphite 0.12% (less than 0.2%), the reaction is complete.
[0035] (2) Add 100 milliliters of methylene chloride and 78.7 grams of p-toluenesulfonyl chloride to the above reaction solution, cool down to 5° C., add 63.8 grams of 30% liquid caustic soda dropwise, the reaction is exothermic, and keep the dropping temperature between 5-10° C. , after adding in 1 hour, after the dropwise addition, keep warm at 10°C for 2 hours, take a sample test, the reaction of p-toluenesulfonyl chloride 0.15% (less than 0.2%) is completed, stand still, separate layers, add 50 ml of dichloromethane...
Embodiment 2
[0048] (1) In a 500 ml three-necked flask, add 55 grams of 37% formaldehyde solution, 3.0 grams of sodium carbonate, cool down to 0°C, add 60 grams of diethyl phosphite dropwise, and keep the temperature during the dropping process between 0-5°C , The addition was completed in 2 hours, and after the addition was completed, the reaction solution was obtained by incubating at 2°C for 2 hours. Sampling detection, diethyl phosphite 0.11% (less than 0.2%), the reaction is complete.
[0049] (2) Add 100 milliliters of methylene chloride and 78.7 grams of p-toluenesulfonyl chloride to the above reaction solution, cool down to 5° C., add 63.8 grams of 30% liquid caustic soda dropwise, the reaction is exothermic, and keep the dropping temperature between 5-10° C. , Added in 1 hour, the dropwise addition was completed and kept at 10°C for 2 hours, sampling was detected, the reaction of p-toluenesulfonyl chloride 0.13% (less than 0.2%) was completed, static, separated, the water layer wa...
Embodiment 3
[0051] 1) In a 500 ml three-necked flask, add 50 grams of 37% formaldehyde solution, 3.0 grams of potassium carbonate, cool down to 0°C, add 60 grams of diethyl phosphite dropwise, and keep the temperature during the dropping process between 0-5°C. The addition was completed in 2 hours, and after the addition, it was incubated at 2°C for 2 hours to obtain a reaction solution. Sampling detection, diethyl phosphite 0.09% (less than 0.2%), the reaction is complete.
[0052](2) Add 100 milliliters of methylene chloride and 78.7 grams of p-toluenesulfonyl chloride to the above reaction solution, cool down to 5° C., add 63.8 grams of 30% liquid caustic soda dropwise, the reaction is exothermic, and keep the dropping temperature between 5-10° C. , after adding in 1 hour, after the dropwise addition, keep warm at 10°C for 2 hours, take a sample test, the reaction of p-toluenesulfonyl chloride 0.18% (less than 0.2%) is completed, stand still, separate layers, add 50 ml of dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com