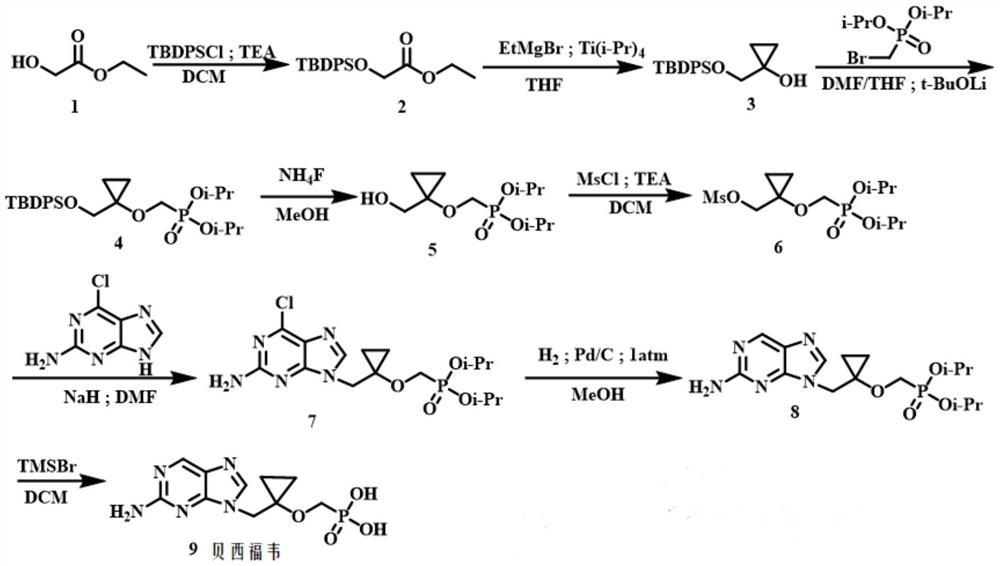
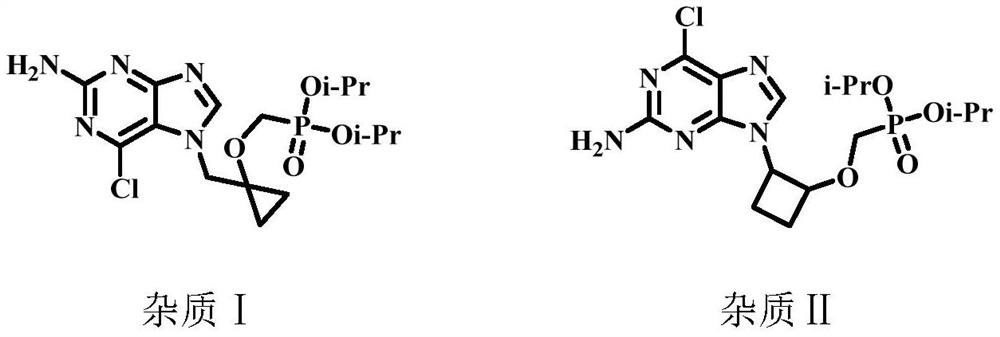
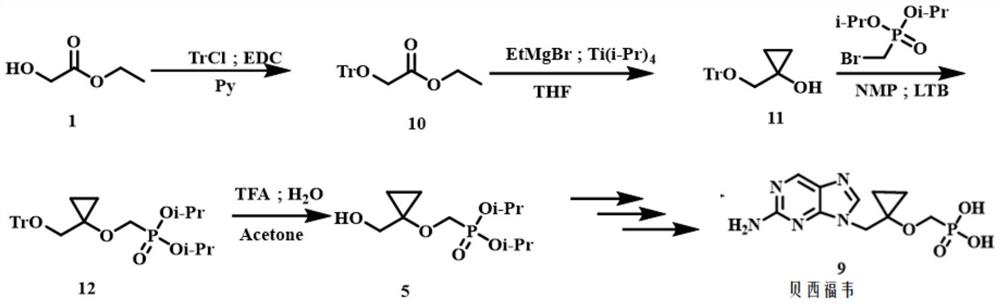
A kind of method for preparing besifovir
A technology of methyl and compound, applied in the field of new intermediates, can solve problems such as high price and increased experimental cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0049] Step 1: Preparation of 2-((tert-butyl(diphenyl)silyl)oxy)ethyl acetate (2):
[0050] 1 (100.0g, 960.52mmol) and imidazole (77.3g, 1.15mol) were dissolved in dichloromethane (1.5L), and tert-butyldiphenylchlorosilane was slowly added dropwise with a constant pressure dropping funnel under ice-cooling conditions (264.0 g, 960.52 mmol). After the dropwise addition was completed, it was transferred to room temperature for 16 h. Filtrate, wash the filtrate with 1.0mol / L hydrochloric acid solution (300ml×2), wash the dichloromethane layer with saturated sodium bicarbonate solution (100ml×2), dry over anhydrous sodium sulfate, and concentrate to give white transparent liquid 2 (297.7g ,91%). ESI-MS(m / z):365.1564[M+Na] + ; 1 H NMR (300MHz, CDCl3) δ: 7.87~7.76 (m, 4H), 7.47 (q, J = 5.4Hz, 6H), 4.35 (s, 2H), 4.22 (q, J = 7.1Hz, 2H), 1.28 (t,J=7.1Hz,3H),1.21(s,9H); 13 CNMR (75MHz, CDCl3) δ: 171.2, 135.7, 132.9, 130.0, 127.9, 62.4, 60.7, 26.8, 19.4, 14.2.
[0051] Step 2: Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com