PMKRN1 (porcine Makorin ring finger protein 1) gene-knockout porcine somatic cell, method for preparing same and application of pMKRN1 gene-knockout porcine somatic cell
A gene knockout and somatic cell technology, applied in the fields of botany equipment and methods, biochemical equipment and methods, cells modified by introducing foreign genetic material, etc., can solve problems that have not been studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] The present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. The examples are by way of illustration, not limitation, of the invention.
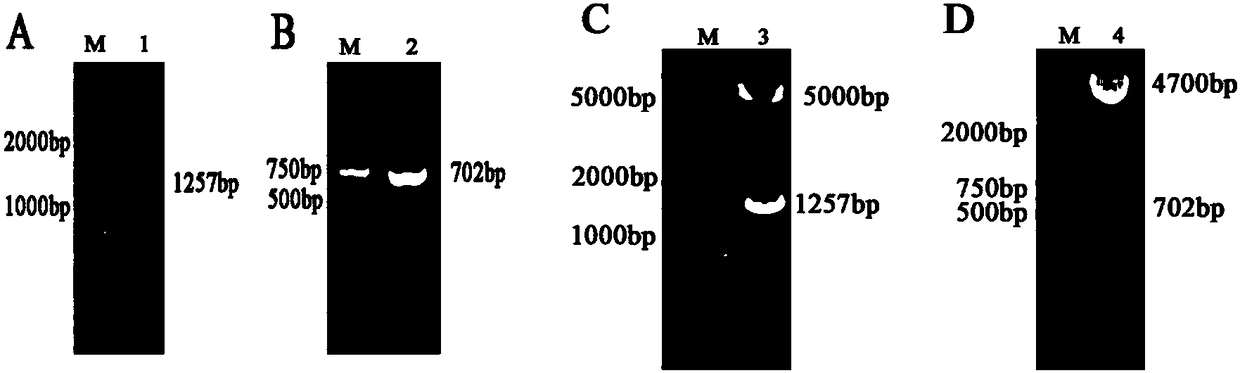
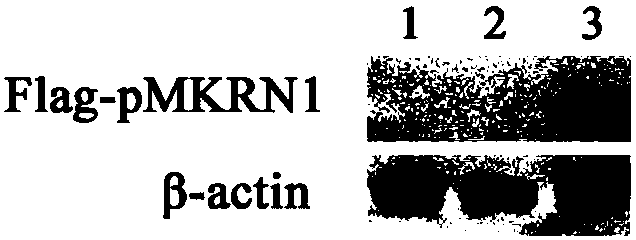
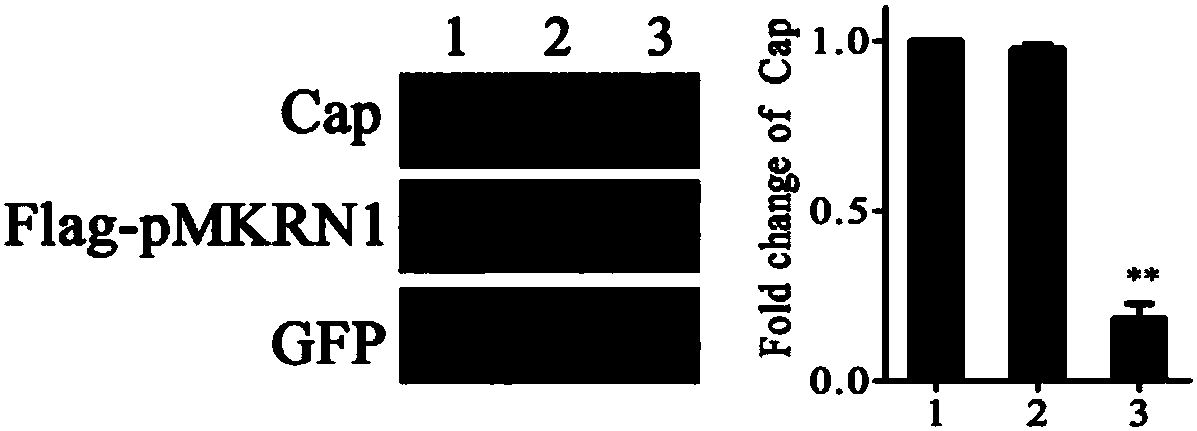
[0035] The present invention detects the effect of pMKRN1 on Cap degradation by co-expressing porcine MKRN1 (pMKRN1) and PCV2Cap; simultaneously constructs pMKRN1 overexpression cells and knockout cell lines to verify the effect of pMKRN1 on PCV2Cap degradation, and utilizes knockout cells to realize PCV2 virus in the host ( For example, high-speed replication in PK-15 cells).
[0036] (1) Construction of pMKRN1 and PCV2Cap eukaryotic expression vectors
[0037] Design the amplification primers for pMKRN1 and PCV2Cap:
[0038] pMKRN1 upstream primer P1: 5'-CCG CTCGAG ATGGATTACAAGGATGACGACGATAAGCATGGGGTTTGTAAGGAAG-3' (the underlined position indicates the Xhol restriction endonuclease recognition sequence, and the italicized position indicates the Flag tag);
[0039...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com