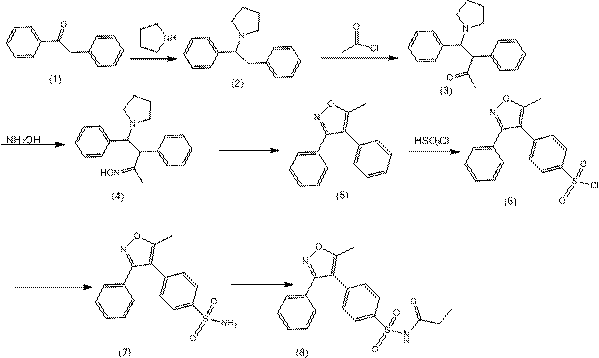
Method for synthesizing parecoxib
A synthesis method, parecoxib technology, applied in the field of parecoxib synthesis, can solve the problems of low yield, complex synthesis process of parecoxib, difficulty in separation, etc., to reduce production cost, increase yield, The effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] According to the ratio of raw materials, that is, the amount of trifluoromethanesulfonic acid is 0.08 (based on the molar number of benzophenone oxime as 1), and the amount of solvent used for acetonitrile and tetrahydrofuran (the volume ratio of the two is 1:1) is 3ml ( The molar dosage of benzophenone oxime is 1 mmol), and the dosage of p-sulfophenylpropyne is 0.5 (the molar dosage of benzophenone oxime is 1). Add magnetons into the three-necked flask, put in p-sulfophenylpropyne, benzophenone oxime, trifluoromethanesulfonic acid, acetonitrile and tetrahydrofuran, and turn on the magnetic stirrer. React at 60°C. Use TLC plate and GC-MS to monitor, after the reaction is complete, cool naturally, filter, and rotate to evaporate the solvent, and finally use n-hexane and ethyl acetate in an appropriate proportion as mobile phase, and purify and separate the pure product by column chromatography, which is 5-Methyl-3-phenyl-4-(4'-sulfo)phenylisoxazole.
[0030] Add 5-meth...
Embodiment 2
[0033] According to the ratio of raw materials, that is, the amount of trifluoromethanesulfonic acid is 0.12 (based on the molar number of benzophenone oxime as 1), and the amount of solvent used for acetonitrile and tetrahydrofuran (the volume ratio of the two is 2:1) is 7ml ( The molar dosage of benzophenone oxime is 1 mmol), and the dosage of p-sulfophenylpropyne is 0.9 (the molar dosage of benzophenone oxime is 1). Add magnetons into the three-necked flask, put in p-sulfophenylpropyne, benzophenone oxime, trifluoromethanesulfonic acid, acetonitrile and tetrahydrofuran, and turn on the magnetic stirrer. React at 80°C. Use TLC plate and GC-MS to monitor, after the reaction is complete, cool naturally, filter, and rotate to evaporate the solvent, and finally use n-hexane and ethyl acetate in an appropriate proportion as mobile phase, and purify and separate the pure product by column chromatography, which is 5-Methyl-3-phenyl-4-(4'-sulfo)phenylisoxazole.
[0034] Add 5-meth...
Embodiment 3
[0037] According to the ratio of raw materials, that is, the amount of trifluoromethanesulfonic acid is 0.12 (based on the molar number of benzophenone oxime as 1), and the amount of solvent used for acetonitrile and tetrahydrofuran (the volume ratio of the two is 2:1) is 3ml ( The molar dosage of benzophenone oxime is 1 mmol), and the dosage of p-sulfophenylpropyne is 0.5 (the molar dosage of benzophenone oxime is 1). Add magnetons into the three-necked flask, put in p-sulfophenylpropyne, benzophenone oxime, trifluoromethanesulfonic acid, acetonitrile and tetrahydrofuran, and turn on the magnetic stirrer. React at 70°C. Use TLC plate and GC-MS to monitor, after the reaction is complete, cool naturally, filter, and rotate to evaporate the solvent, and finally use n-hexane and ethyl acetate in an appropriate proportion as mobile phase, and purify and separate the pure product by column chromatography, which is 5-Methyl-3-phenyl-4-(4'-sulfo)phenylisoxazole.
[0038] Add 5-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com