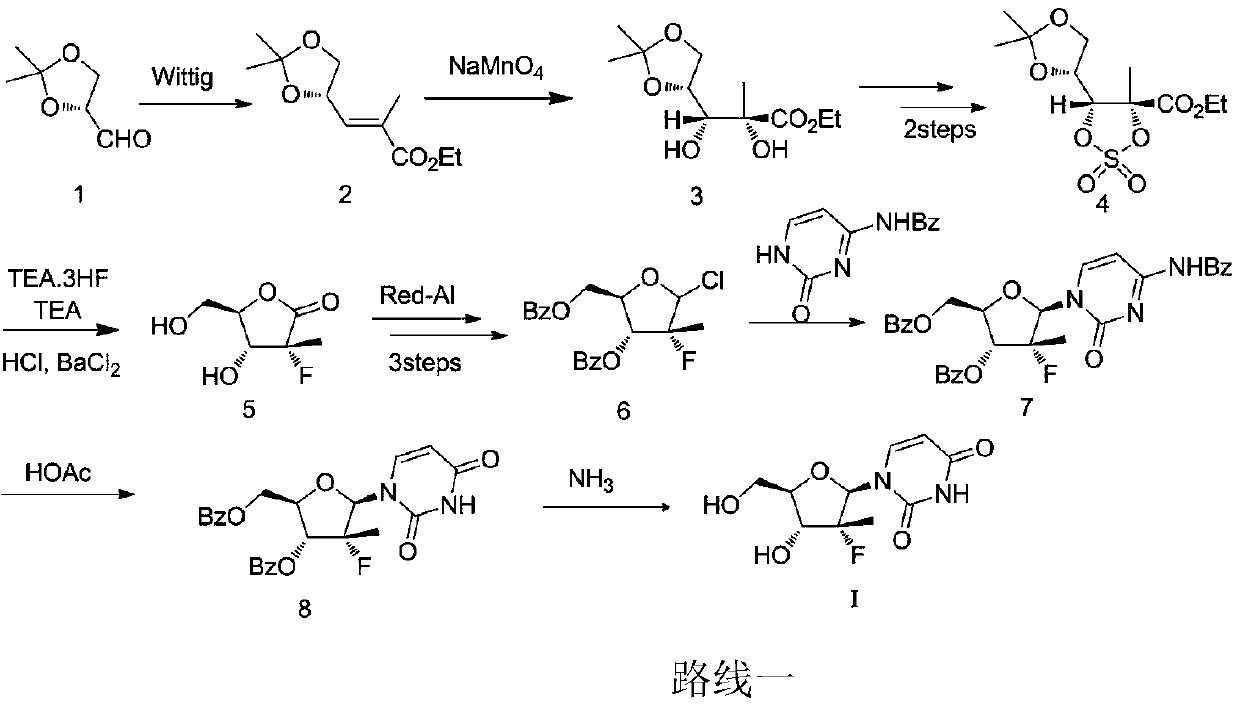
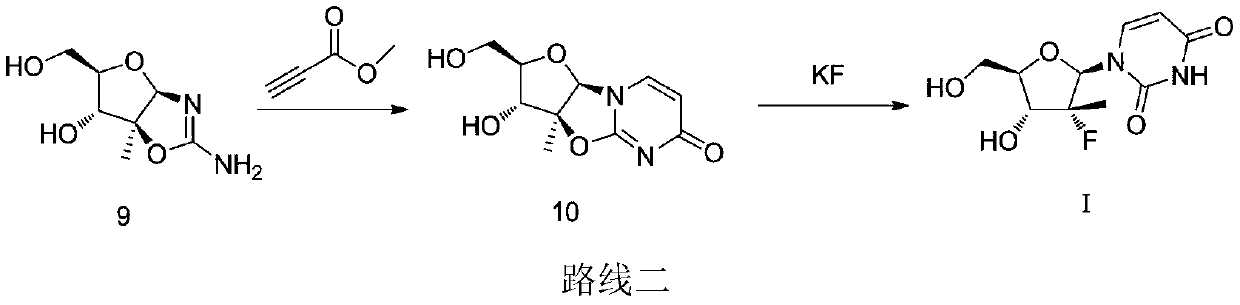
A kind of synthetic technique of (2'r)-2'-deoxy-2'-fluoro-2'-methyluridine
A synthesis process and technology of methyl uridine, applied in the directions of esterification saccharides, sugar derivatives, organic chemistry, etc., can solve the problems of expensive reducing reagents, expensive raw materials, long synthesis routes, etc., and achieve easy control and synthesis of process conditions. The effect of easy process and improved product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
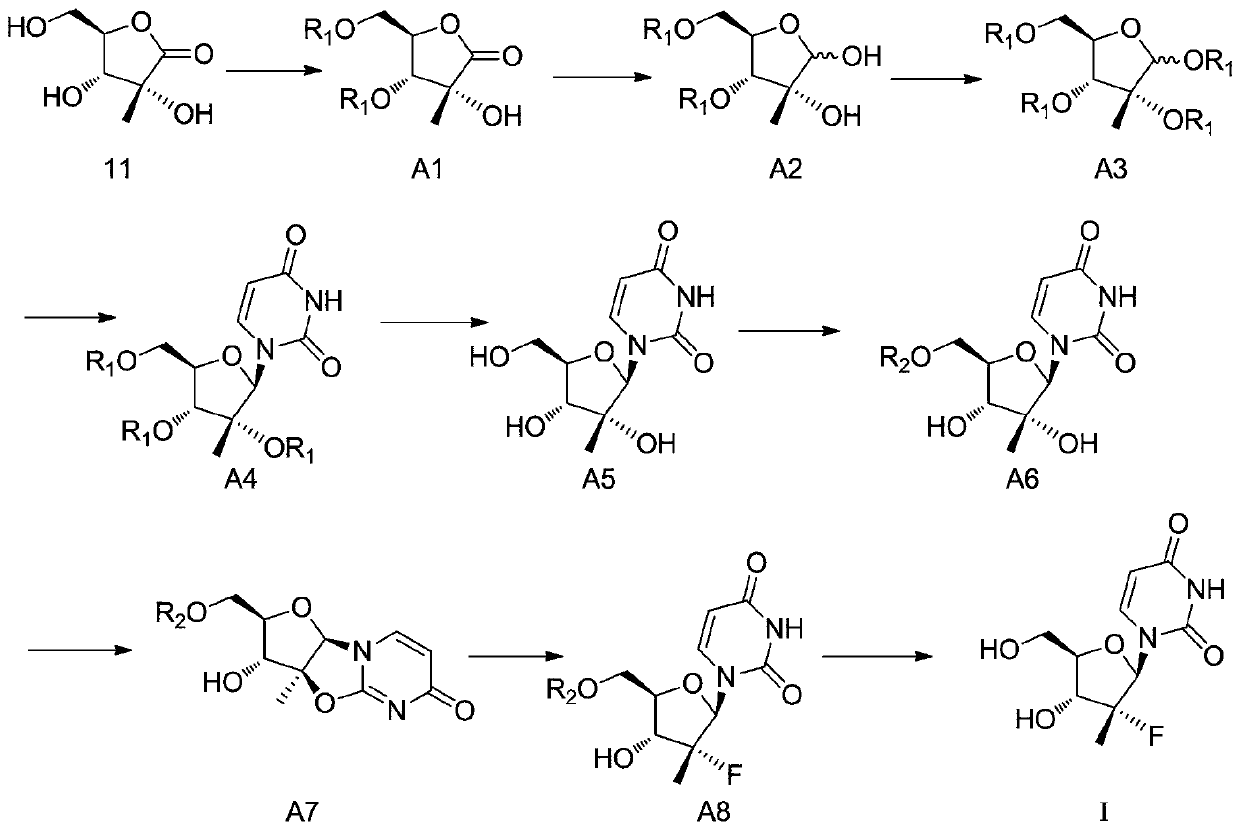
[0042] Embodiment 1: A synthesis process of (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, comprising the following steps:
[0043] Step 1, the synthesis of compound A1: add 35g of compound 11, 61g of protective reagent R1 and 200mL of dichloromethane into the reaction flask, R1 is benzoyl chloride, cool down in an ice bath, stir, slowly add 35g of pyridine dropwise, after the dropwise addition, The reaction was stirred at 0° C. for 5 hours, washed with water, and spin-dried to obtain compound A1 with a yield of 90%.
[0044] Step 2, the synthesis of compound A2: add 72g of compound A1 and 350mL of the first organic solvent successively in the reactor, the first organic solvent is methylene chloride, add 5g of glacial acetic acid and 6g of
[0045]The reducing agent, the reducing agent is sodium borohydride, the mass ratio of the reducing agent and compound 1 is 0.5:1, the temperature is controlled at 10°C, the reaction is stirred until the raw materials disappear, and the acid is...
Embodiment 2
[0068] Example 2: A synthesis process of (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, the difference from Example 1 is that the first organic The solvent is 1,2 dichloroethane.
Embodiment 3
[0069] Example 3: A synthesis process of (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, the difference from Example 1 is that the first organic The solvent is methanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com