Synthetic method for (R)-N-tert-butoxycarbonyl-3-hydroxymethylpiperidine
A technology of hydroxymethylpiperidine and tert-butoxycarbonyl, which is applied in the field of synthesis of -N-tert-butoxycarbonyl-3-hydroxymethylpiperidine, and can solve problems such as lack of scalability, complicated methods, and harsh conditions. problems, to achieve the effects of improving ease of operation and convenience, ensuring yield and purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
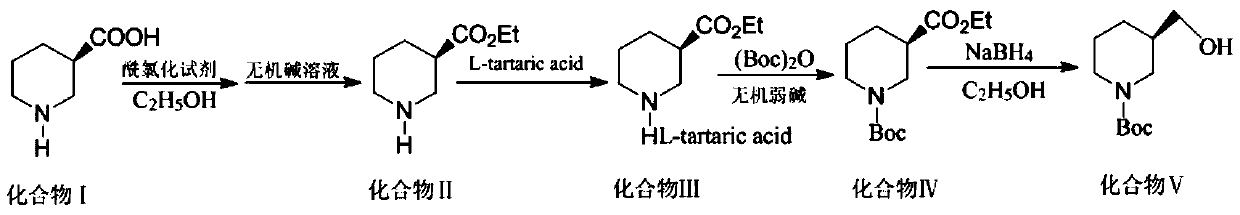
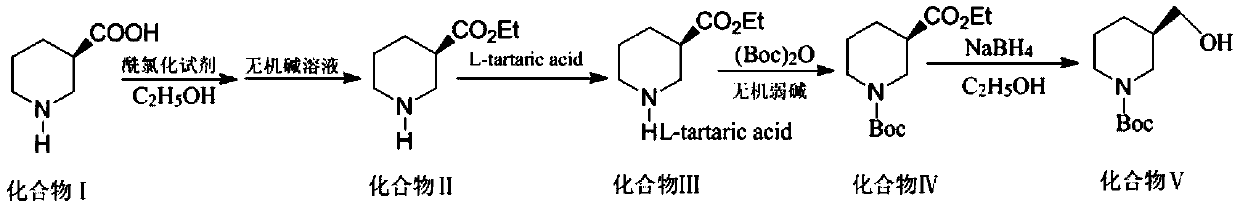
[0023] Prepare (R)-N-tert-butoxycarbonyl-3-hydroxymethylpiperidine by using the process of the present invention, comprising the steps of:
[0024] Step S1: To synthesize ethyl 3-piperidinecarboxylate (compound II), add 50 mL of absolute ethanol and 12.9 g (0.1 mol) of 3-piperidinecarboxylic acid (compound I) into the reaction flask, and control the temperature at 0°C; In the state, add thionyl chloride 13.1g (0.105mol) dropwise to the reaction flask, heat to reflux temperature of 70°C, reflux reaction until the reaction material is clear, and the reaction ends; the excess ethanol is evaporated under reduced pressure, cooled to room temperature; Adjust the pH to 12 with 5% sodium hydroxide aqueous solution, then add 25mL dichloromethane for extraction, and then extract the aqueous layer twice with dichloromethane, combine the organic phases, and pass the organic phases through saturated sodium bicarbonate, purified water, and saturated brine successively. Wash, dry with anhydr...
Embodiment 2
[0030] Prepare (R)-N-tert-butoxycarbonyl-3-hydroxymethylpiperidine by the process of the present invention, comprising the steps of:
[0031] Step S1: To synthesize ethyl 3-piperidinecarboxylate (compound II), add 50 mL of absolute ethanol and 12.9 g (0.1 mol) of 3-piperidinecarboxylic acid (compound I) into the reaction flask, and control the temperature at 0°C; 15 g (0.12 mol) of thionyl chloride was added dropwise to the reaction flask, heated to a reflux temperature of 70° C., refluxed until the reaction material was clear, and the reaction ended; excess ethanol was evaporated under reduced pressure and cooled to room temperature; % Potassium hydroxide aqueous solution to adjust the pH to 12, then add 25mL dichloromethane for extraction, and then extract the aqueous layer twice with dichloromethane, combine the organic phases, and wash the organic phases successively with saturated sodium bicarbonate, purified water, and saturated brine , dried with anhydrous sodium sulfat...
Embodiment 3
[0037] Prepare (R)-N-tert-butoxycarbonyl-3-hydroxymethylpiperidine by the process of the present invention, comprising the steps of:
[0038] Step S1: To synthesize ethyl 3-piperidinecarboxylate (compound II), add 50 mL of absolute ethanol and 12.9 g (0.1 mol) of 3-piperidinecarboxylic acid (compound I) into the reaction flask, and control the temperature at 0°C; 17.81g (0.13mol) of phosphorus trichloride was added dropwise to the reaction flask under the condition of Adjust the pH to 13 with 5% sodium hydroxide aqueous solution, then add 25mL dichloromethane for extraction, and then extract the aqueous layer twice with dichloromethane, combine the organic phases, and pass the organic phases through saturated sodium bicarbonate, purified water, and saturated brine successively. Wash, dry with anhydrous sodium sulfate for 10 h, filter with suction, and concentrate the filtrate under reduced pressure to obtain 14.2 g of oily liquid (Compound II), the yield of this step is 90% (b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com