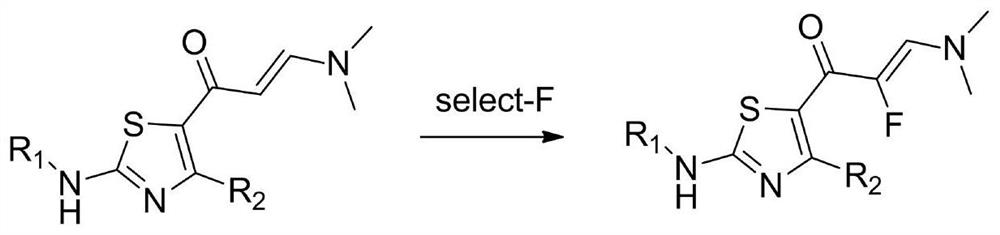
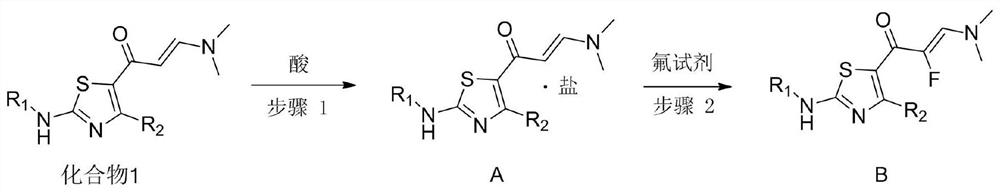
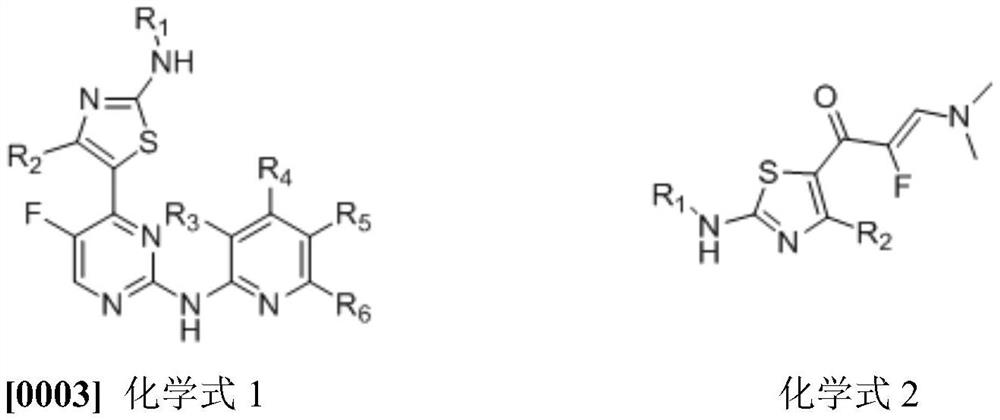
An important synthesis process of fluorine intermediate
A kind of synthesis technique, the technology of intermediate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: An important synthetic process for fluorine intermediates, prepared through the following steps:
[0029] (1) Add 400g compound 1 and 2.5L methanol in the reaction flask, cool down in an ice bath, stir, and slowly add dropwise a solution composed of 200g trifluoroacetic acid and an organic solvent for 40min. The organic solvent is methanol. After the dropwise addition, at 25 Stirred under the condition of ℃ for 6 hours, spin-dried to obtain 557g of compound A shown in chemical formula A, the yield was 99%, LCMS (M+H): 226.1;
[0030] (2) Add 550g of Compound A and 3.0L of organic solvent in sequence in the reactor. The organic solvent is methanol. Add 314g of fluorine reagent in batches under ice bath. The fluorine reagent is 1-chloromethyl-4-fluoro-1,4- Diazotization of bicyclic 2.2.2-octane bis(tetrafluoroborate), naturally warming up to 20°C, stirring for 1 hour, monitoring the reaction by TLC until the raw materials disappeared, adding ammonia methanol so...
Embodiment 2
[0032] Example 2: An important synthetic process for fluorine intermediates, the difference from Example 1 is that R1 and R2 are both ethyl groups.
Embodiment 3
[0033] Example 3: An important synthetic process for fluorine intermediates, the difference from Example 1 is that R1 and R2 are both propyl groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com