Synthesis process of ketorolac
A synthesis process, a technology for ketorolac, applied in the direction of organic chemistry, etc., can solve the problems of difficult process wastewater treatment, unfavorable environmental protection, dark product color, etc., and achieves easy control of process conditions, increased process production safety, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
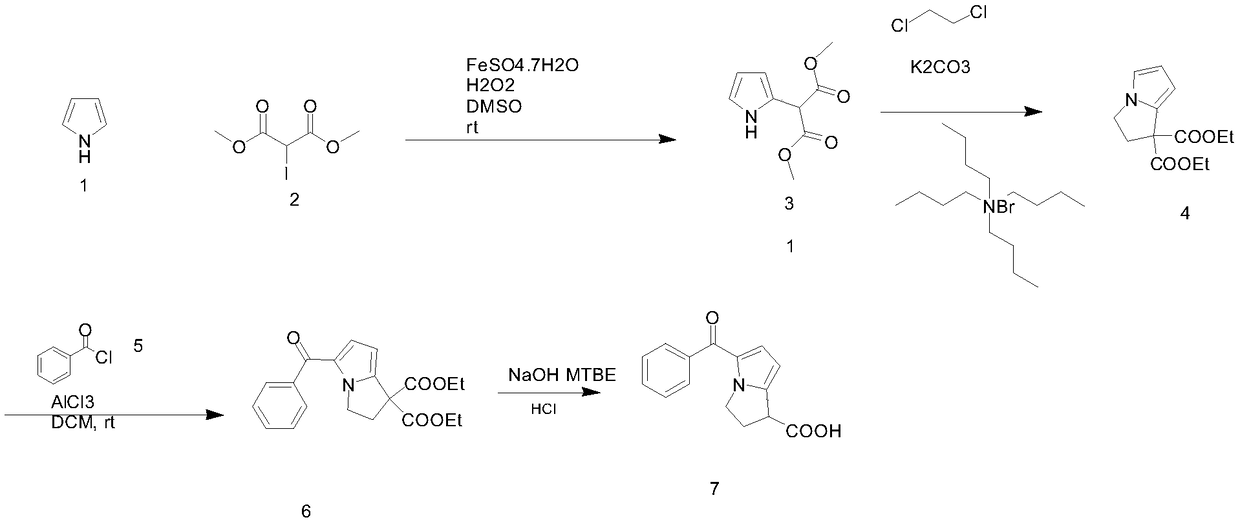
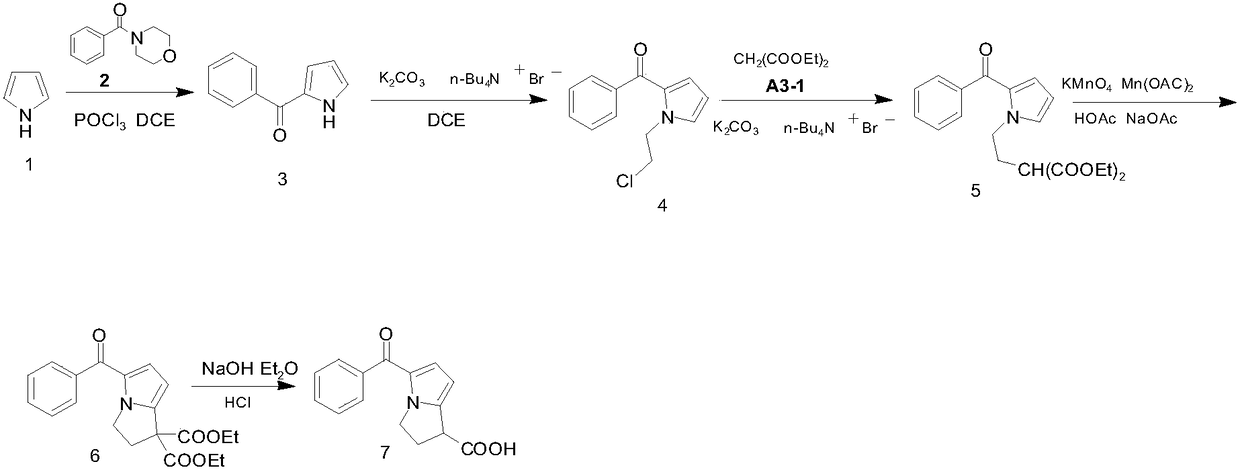
[0036] Embodiment 1: a kind of synthetic technique of ketorolac, press figure 1 The reaction formula in carries out, and it comprises the following steps:
[0037] Step 1, the synthesis of compound 3: add 5 equivalents of pyrrole, 1 equivalent of dimethyl iodomalonate to the reaction flask, then add dimethyl sulfoxide and 0.1 equivalent of iron salt catalyst, the iron salt catalyst is ferrous sulfate heptahydrate, The weight ratio of dimethyl sulfoxide to dimethyl iodomalonate is 2:1. Add 5 equivalents of oxidant dropwise to the flask under stirring at room temperature. The oxidant is hydrogen peroxide. The dropwise addition time is 0.5 hours. After the dropwise addition is complete, continue to stir React for 0.5 hours, then add 5 equivalents of dichloroethane, separate the phases, wash the organic phase twice with 10ml of water, dry with a desiccant, the desiccant is anhydrous sodium sulfate, filter, and concentrate the filtrate until no liquid flows out. Distill the remain...
Embodiment 2
[0042] Embodiment 2: a kind of synthetic technique of ketorolac, differs from embodiment 1 in that, comprises the following steps:
[0043] Step 1, the synthesis of compound 3: add 13 equivalents of pyrrole, 1 equivalent of dimethyl iodomalonate to the reaction flask, then add dimethyl sulfoxide and 0.3 equivalents of iron salt catalyst, the iron salt catalyst is ferrous sulfate heptahydrate, The weight ratio of dimethyl sulfoxide to dimethyl iodomalonate is 3:1. Add 10 equivalents of oxidant dropwise into the flask under stirring at room temperature. The oxidant is hydrogen peroxide. The dropwise addition time is 0.8 hours. After the dropwise addition is complete, continue to stir React for 0.8 hours, then add 10 equivalents of dichloroethane, separate the phases, wash the organic phase twice with 10ml of water, dry with a desiccant, the desiccant is anhydrous sodium sulfate, filter, and concentrate the filtrate until no liquid flows out. Distill the remaining pyrrole under r...
Embodiment 3
[0047] Embodiment 3: a kind of synthetic technique of ketorolac, differs from embodiment 1 in that, comprises the following steps:
[0048] Step 1, the synthesis of compound 3: add 20 equivalents of pyrrole, 1 equivalent of dimethyl iodomalonate to the reaction flask, then add dimethyl sulfoxide and 0.5 equivalents of iron salt catalyst, the iron salt catalyst is ferrous sulfate heptahydrate, The weight ratio of dimethyl sulfoxide to dimethyl iodomalonate is 5:1. Add 15 equivalents of oxidant dropwise into the flask under stirring at room temperature. The oxidant is hydrogen peroxide. The dropwise addition time is 1 hour. After the dropwise addition is complete, continue to stir React for 1 hour, then add 15 equivalents of dichloroethane, separate the phases, wash the organic phase twice with 10ml of water, dry with a desiccant, the desiccant is anhydrous sodium sulfate, filter, and concentrate the filtrate until no liquid flows out. Distill the remaining pyrrole under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com