Treatment method for separating and extracting nickel, cobalt, magnesium and iron from laterite nickel ores
A technology of lateritic nickel ore and treatment method, which is applied to the improvement of process efficiency, photography technology, instruments, etc., can solve the problems of complex and cumbersome operation methods, incomplete precipitation of cobalt, and lake drying up and death, and achieve high metal separation and extraction rates, Shorten the leaching time and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
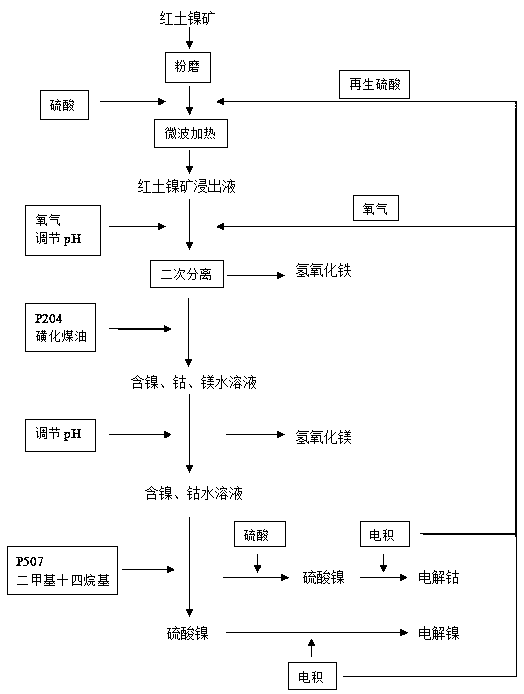
[0038] (1) Pretreatment of laterite nickel ore: Put the laterite nickel ore into the material bed for grinding to make the soil powdery, add 3mol / L sulfuric acid, the solid-to-liquid ratio is 1:3.5, and then heat it with microwave 700W for 2 hours to obtain the leachate I, laterite The content of main valuable metals in the nickel ore leachate I was measured to be nickel: 4.52g / L, cobalt: 0.28 g / L, iron: 95.98 g / L, magnesium 6.11 g / L;
[0039] (2) Secondary separation of iron: put the leachate I into the reactor, pump oxygen to pressurize to 0.8MPa, control the temperature at 55°C, and adjust the pH of the leachate I to 2 to hydrolyze and precipitate the ferric iron into ferric hydroxide to obtain the leachate II; The leach solution II flows into the next reaction kettle, and the pH of the leach solution II is precisely adjusted to 3.2 to completely precipitate the ferric iron to obtain the leach solution III;
[0040] (3) Extraction of other trace metals: adding 1 part of P20...
Embodiment 2
[0047] (1) Pretreatment of laterite nickel ore: Put the laterite nickel ore into the material bed for grinding to make the soil powdery, add 3mol / L sulfuric acid, the solid-liquid ratio is 1:3, and then heat it with microwave 800W for 2.5h to obtain the leachate I. The content of main valuable metals in the lateritic nickel ore leachate I was measured to be nickel: 5.18 g / L, cobalt: 0.22 g / L, iron: 93.65 g / L, and magnesium: 6.89 g / L.
[0048] (2) Secondary separation of iron: Put the leachate I into the reactor, pump oxygen to pressurize to 0.6MPa, control the temperature at 60°C, and adjust the pH of the leachate I to 3.0 to hydrolyze and precipitate the ferric iron into ferric hydroxide to obtain the leachate II; The leach solution II flows into the next reaction kettle, and the pH of the leach solution II is precisely adjusted to 3.2 to completely precipitate the ferric iron to obtain the leach solution III;
[0049] (3) Extraction of other trace metals: add 1 part of P204 ...
Embodiment 3
[0056] (1) Pretreatment of laterite nickel ore: Put the laterite nickel ore into the material bed for grinding to make the soil powdery, add 3mol / L sulfuric acid, the solid-liquid ratio is 1:4, and then heat it with microwave 900W for 1.5h to obtain the leachate I. The content of main valuable metals in the lateritic nickel ore leachate I was measured as nickel: 4.86g / L, cobalt: 0.19 g / L, iron: 97.38 g / L, and magnesium: 6.35 g / L.
[0057] (2) Secondary separation of iron: put the leachate I into the reactor, pump oxygen to pressurize to 1MPa, control the temperature to 70°C, and adjust the pH of the leachate I to 2.0 to hydrolyze and precipitate the ferric iron into ferric hydroxide to obtain the leachate II; II flows into the next reactor, and the pH of the leachate II is precisely adjusted to 3.2 to completely precipitate the ferric iron to obtain the leachate III;
[0058] (3) Extraction of other trace metals: adding 1 part of P204 and 2 parts of sulfonated kerosene to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com