Leaching system and leaching method for valuable metal in waste lithium battery
A technology for waste lithium batteries and valuable metals, applied in battery recycling, waste collector recycling, recycling technology, etc., to achieve enhanced leaching, high leaching rate, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the leaching of lithium, cobalt in the waste lithium cobalt oxide battery
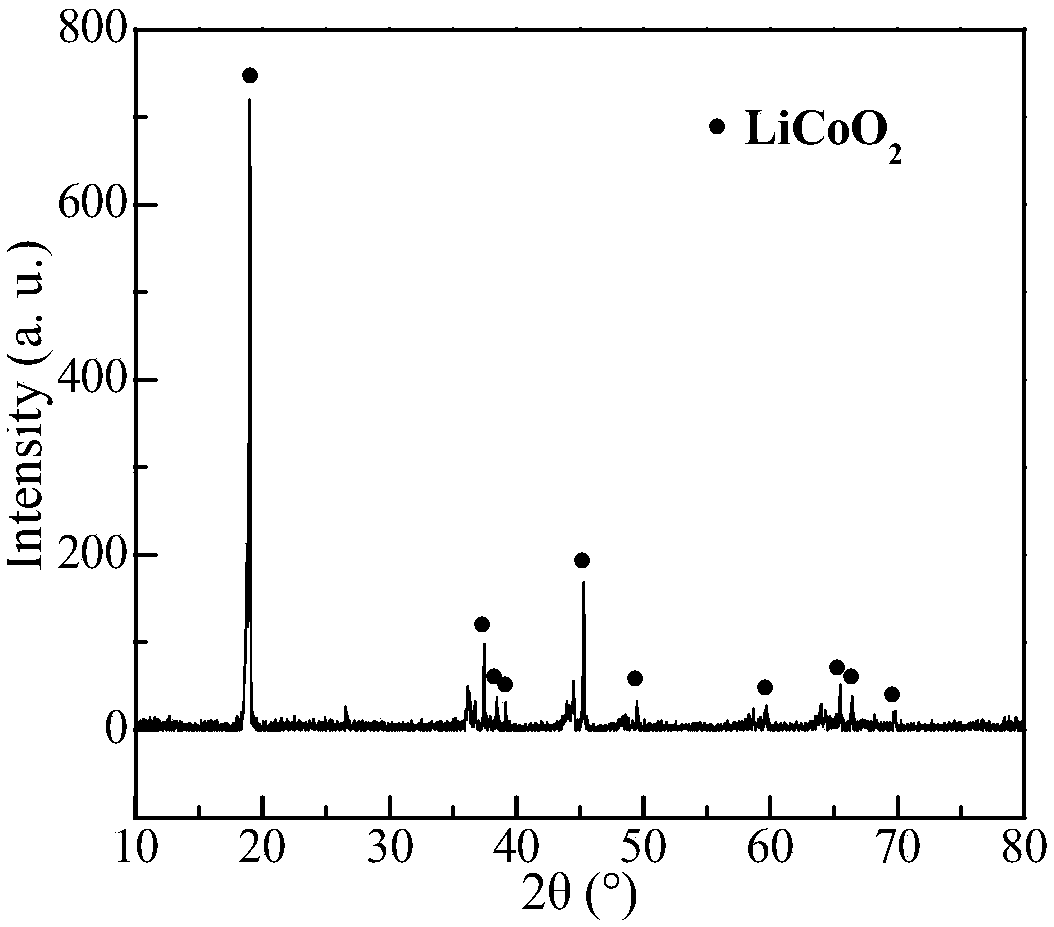
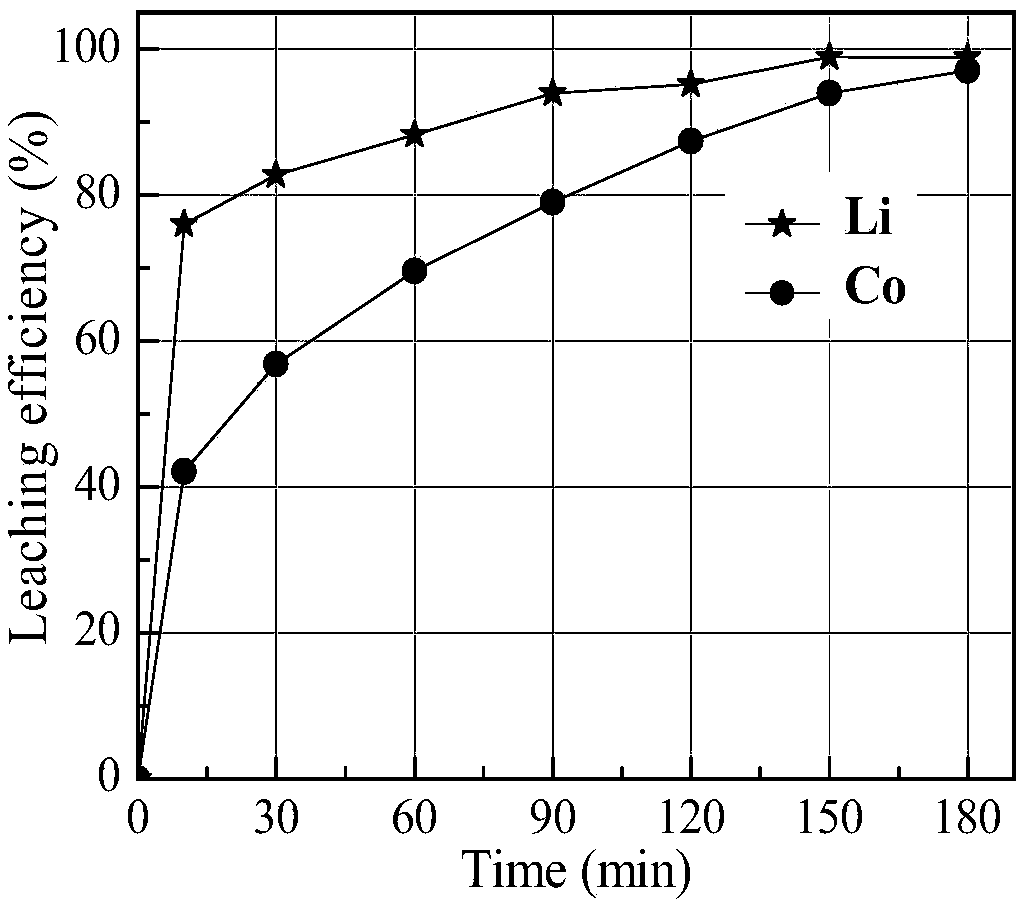
[0033] After pretreatment such as dismantling and crushing of waste lithium cobalt oxide batteries, the positive electrode powder is separated. The positive electrode powder contains 5.37% Li and 57.73% Co, mainly LiCoO 2 Morphological existence ( figure 1 ). Prepare 100mL sulfamic acid-glucose mixed solution (sulfamic acid concentration 1.5mol / L, glucose 50g / L) and pour it into a 250mL Erlenmeyer flask. After the leaching solution was preheated to 80° C., 5 g of positive electrode powder (solid-to-liquid ratio 50 g / L) was added, and the leaching reaction was carried out under the condition of magnetic stirring (600 rpm). Under different leaching time conditions, the leaching rate of lithium and cobalt in the positive electrode powder of waste lithium cobalt oxide battery changes as follows: figure 2 shown.
Embodiment 2
[0034] Embodiment 2: the leaching of lithium, cobalt, nickel, manganese in the waste ternary lithium battery
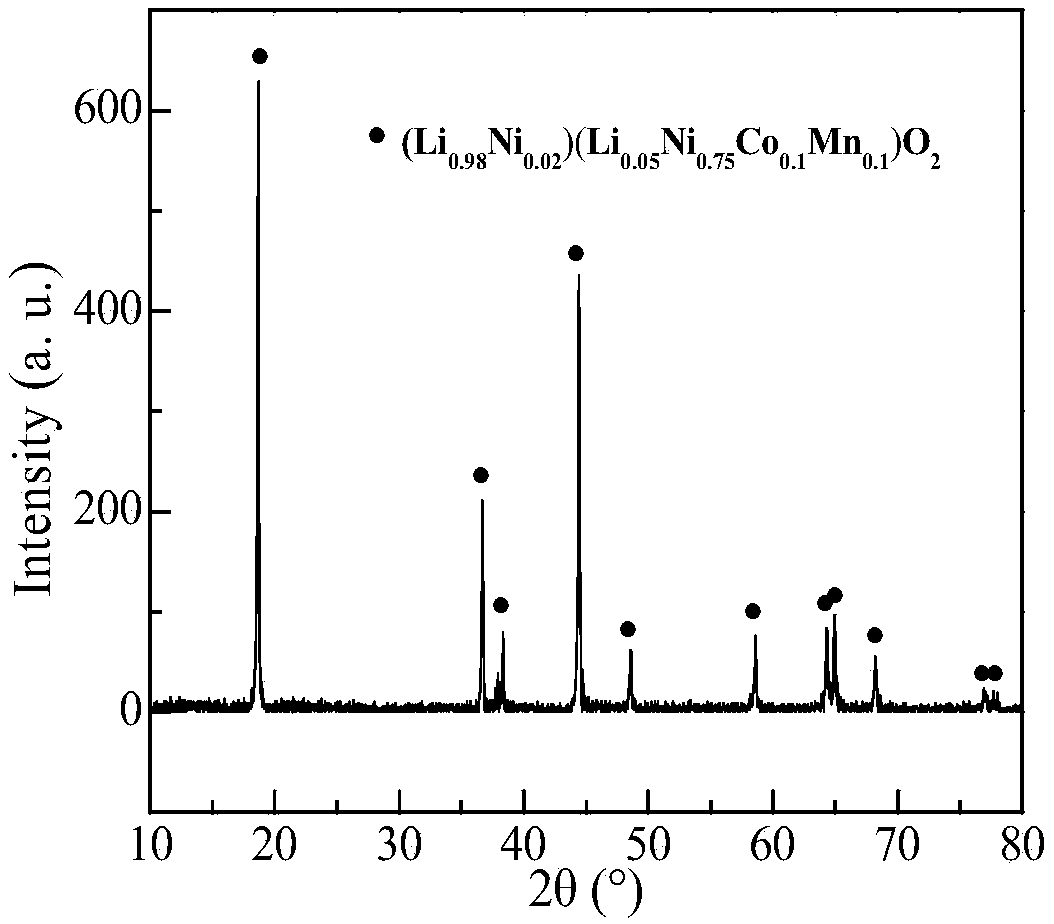
[0035] After the waste ternary lithium battery is disassembled, crushed and other pretreatments, the positive electrode powder is separated. The positive electrode powder contains Li 7.38%, Co14.52%, Ni 35.03%, and Mn 18.63%, mainly in the form of lithium-cobalt-nickel-manganese-oxygen complex ( image 3). Prepare 100mL sulfamic acid-glucose mixed solution (sulfamic acid concentration 1.5mol / L, glucose 40g / L), and pour it into a 250mL Erlenmeyer flask. After the leaching solution was preheated to 80°C, 4g of positive electrode powder (solid-to-liquid ratio: 40g / L) was added, and the leaching reaction was carried out under the condition of magnetic stirring (500rpm). Under different leaching time conditions, the leaching rates of lithium, cobalt, nickel, and manganese in the positive electrode powder of waste ternary lithium batteries change as follows: Figure 4 sh...
Embodiment 3
[0036] Embodiment 3: the leaching of lithium, cobalt, nickel, manganese in the waste ternary lithium battery
[0037] After the waste ternary lithium battery is disassembled, crushed and other pretreatments, the positive electrode powder is separated. The positive electrode powder contains 7.38% Li, 14.52% Co, 35.03% Ni, and 18.63% Mn. Prepare 100mL sulfamic acid-glucose mixed solution (sulfamic acid concentration 1.2mol / L, glucose 50g / L) and pour it into a 250mL Erlenmeyer flask. After the leaching solution was preheated to 85°C, 5g of positive electrode powder (solid-to-liquid ratio: 50g / L) was added, and the leaching reaction was carried out under the condition of magnetic stirring (500rpm). After leaching for 2 hours, the leaching rates of lithium, cobalt, nickel, and manganese in the positive electrode powder of waste ternary lithium batteries were 97.18%, 95.41%, 95.83%, and 95.29%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com