Compound ferrous sulfate sustained release preparation
A slow-release preparation and ferrous sulfate technology, which can be used in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as drug efficacy and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
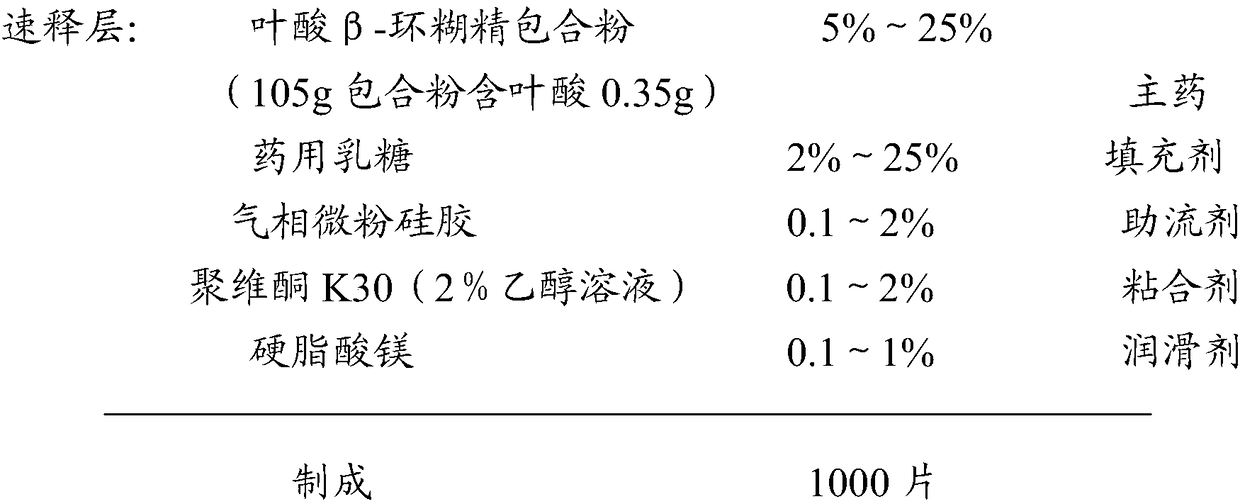
[0091] Embodiment 1 compound ferrous sulfate slow-release preparation
[0092] prescription
[0093]
[0094]
[0095] Weight of slow release layer: weight of quick release layer: 324.48:229.9=1.41:1.
[0096] Preparation method:
[0097] A, the preparation of dry ferrous sulfate
[0098] After crushing medicinal ferrous sulfate (CP2000 standard, containing 7 crystal waters) with a universal grinder, pass through an 80-mesh sieve, dry with hot air at 40-45°C for 36-48 hours, cool and grind with a universal grinder, pass through 100 Mesh sieve, after passing the test (conforming to the standard of BP1988 version about dry ferrous sulfate), it is ready for use.
[0099] B, preparation of slow-release ferrous sulfate particles
[0100] Dry ferrous sulfate and lactose, HPMC k100 Mix microcrystalline cellulose for later use, carmine 60, povidone K 30 Dissolve with 40% (w / v) ethanol, add to the above-mentioned mixed powder in advance, use a one-step granulator to granula...
Embodiment 5~7
[0115] The influence of embodiment 5~7 retarder dosage
[0116] Adopt the method identical with embodiment 1, wherein use the consumption of the retarder of table 4
[0117] The consumption (gram) of table 4 retarder
[0118]
ferrous sulfate
HPMC K100
HPMC K100
HPMC K100
Example 5
74.5
56(10%)
Example 6
74.5
112(20%)
Example 7
74.5
168(30%)
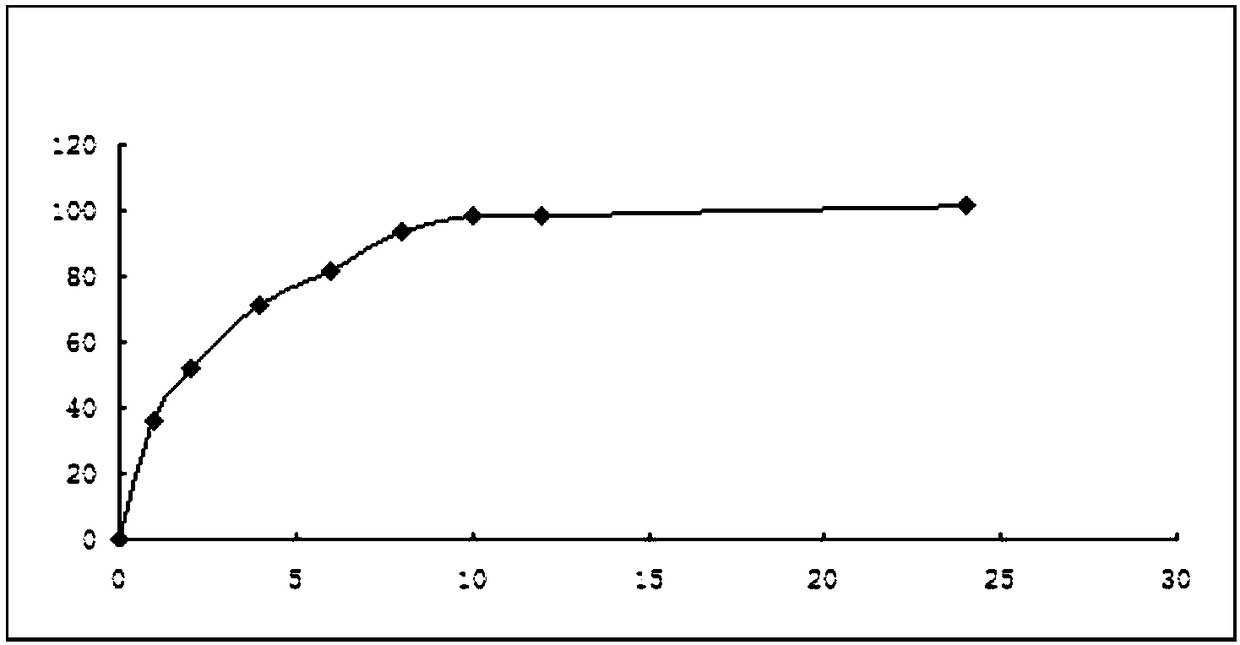
[0119] The tablet prepared in Examples 5-7 is according to the release rate assay method, with 1000ml of 0.1mol hydrochloric acid as the medium, and the rotating speed is 100 rpm; the measurement data are shown in the following table 5:
[0120] Table 5 release measurement data (%)
[0121]
Embodiment 8
[0123] After the slow-release granules and folic acid inclusion granules were prepared according to the method in Example 1, the two granules were uniformly mixed and compressed conventionally to obtain single-layer tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com