Extraction agent for precious metal separation and method for extracting and separating precious metals by using extraction agent
A separation method and precious metal technology, applied in the field of extraction, can solve the problems of limited selection of extraction agents, low extraction and separation efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
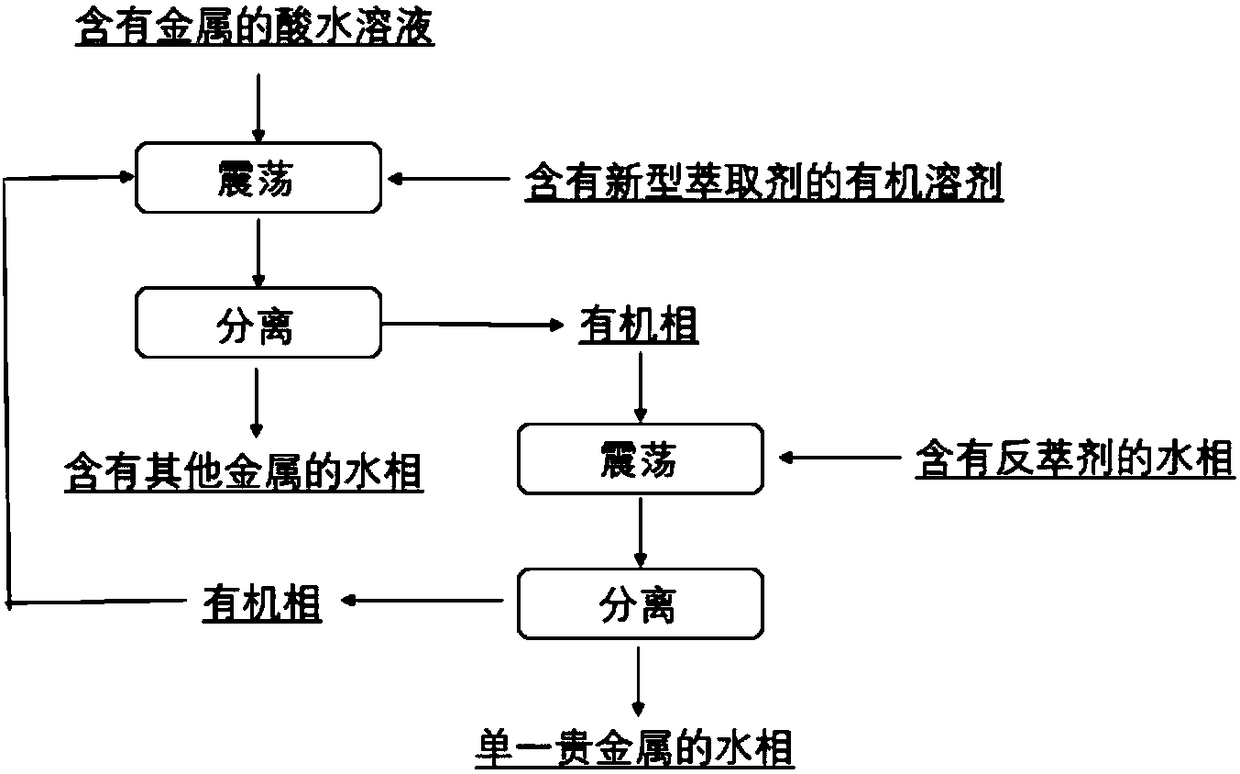
[0049] Among the present invention, the preparation method of described extraction agent, comprises the steps:
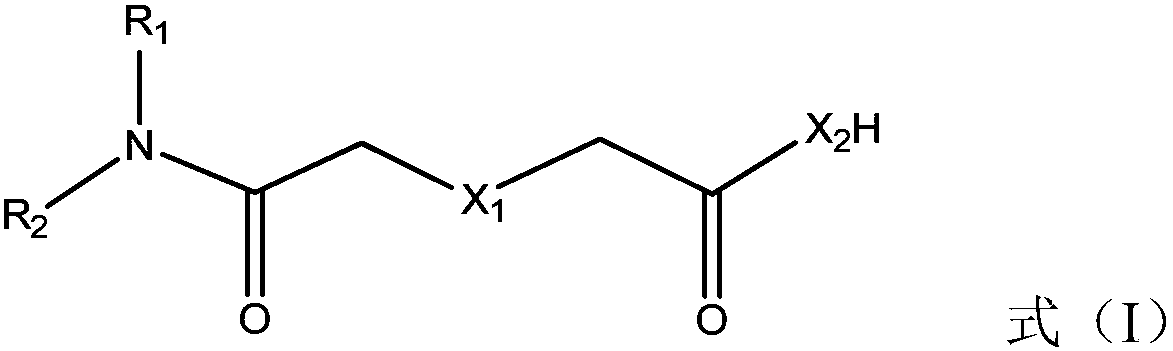
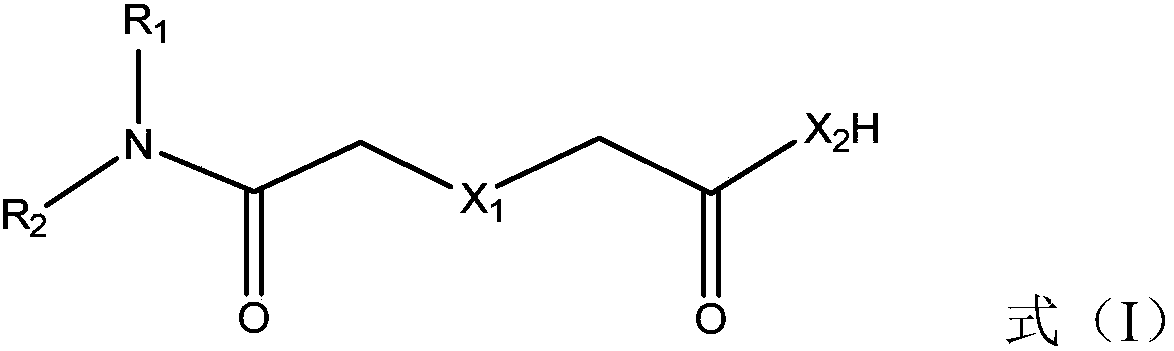
[0050] Compound shown in formula (II) and R 1 R 2 NH is a raw material, and at a certain temperature, reacts to obtain the extraction agent for noble metal separation shown in formula (I);
[0051]
[0052] Among them, X 1 、X 2 , R 1 , R 2 is defined as above.
[0053] In a preferred embodiment of the present invention, the preparation method specifically includes the following steps:
[0054] 1) compound shown in formula (II) and R respectively 1 R 2 NH is dissolved in an organic solvent, where, X 1 、X 2 , R 1 , R 2 as defined above;
[0055] 2) The above two solutions are mixed and reacted to prepare the extractant for separating precious metals represented by formula (I).
[0056] Wherein, the compound represented by formula (II) is diglycolic anhydride.
[0057] Wherein, in step 1), the organic solvent is selected from one or both of dichlorome...
Embodiment 1
[0079] Weigh 1.52g (13.1mmol) diglycolic anhydride and 2.87g (11.9mmol) R 1 R 2 NH(R 1 selected from isooctyl, R 2 selected from isooctyl), which were dissolved in 20mL of dichloromethane organic solvent respectively; the two solutions were mixed and reacted at 25°C; during the reaction, the mixed solution gradually became clear, and when it was completely clear, the reaction Complete (about reaction 12h), make extraction agent shown in formula (I) (wherein, X 1 and x 2 Same as -O-, R 1 and R 2 The same is isooctyl); the prepared extractant is washed with high-purity deionized water to remove impurities, and then magnesium sulfate is added to the washed product to filter to obtain a water-free product, and the processed product is rotated The organic solvent was removed by evaporating and condensing apparatus, and then dried in a vacuum oven at 75°C for 12 hours, so as to obtain a high-purity extractant. Wash the prepared extractant several times with 0.1M hydrochloric ...
Embodiment 2
[0088] Weigh 1.52g (13.1mmol) diglycolic anhydride and 2.87 (11.9mmol) R 1 R 2 NH(R 1 selected from 2-ethylhexyl, R 2 Selected from 2-ethylhexyl), it is dissolved in 20mL dichloromethane organic solvent respectively; Under 25 ℃, react; Reaction 12h), the extraction agent (wherein, X) shown in formula (I) is obtained 1 and x 2 Same as -O-, R 1 and R 2 The same is 2-ethylhexyl); the prepared extractant is washed with high-purity deionized water to remove impurities, and then magnesium sulfate is added to the washed product to filter to obtain a water-free product, and the processed product The organic solvent was removed by a rotary evaporator condenser, and then dried in a vacuum oven at 75° C. for 12 hours to obtain an extractant with higher purity. Wash the prepared extractant several times with 0.1M hydrochloric acid or crystallize with n-hexane to obtain a more pure extractant.
[0089] The purity of the extractant (referred to as extractant E2) prepared by the abov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com