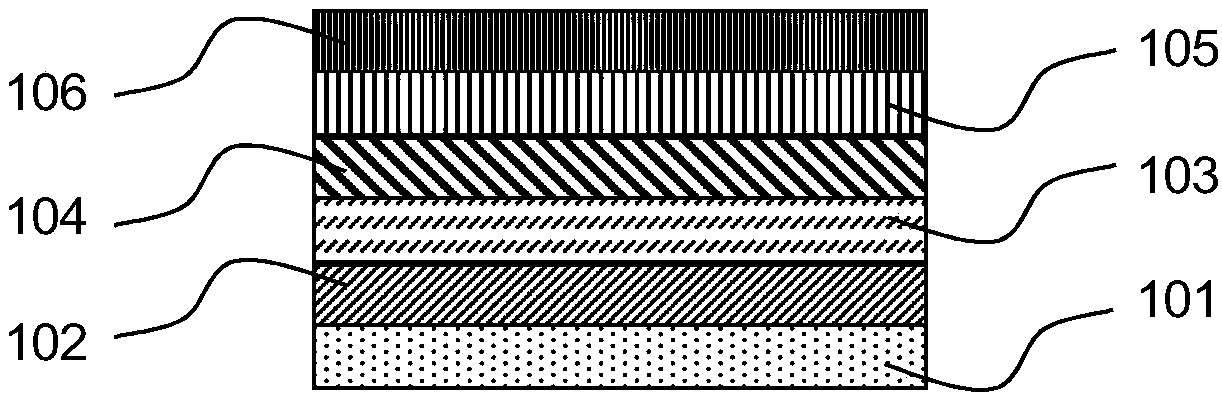
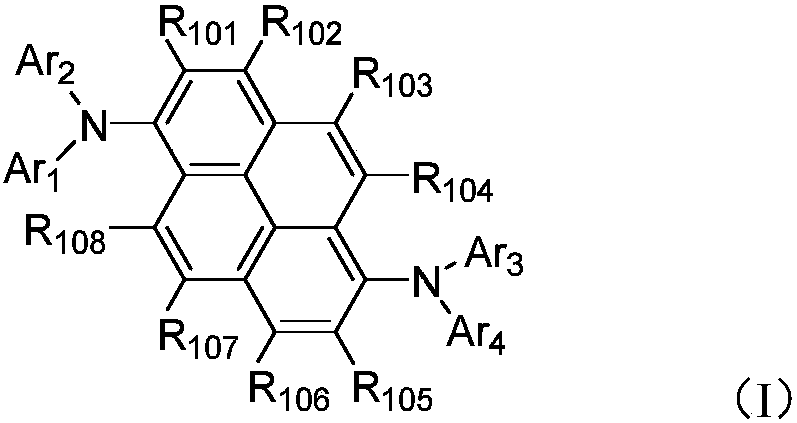
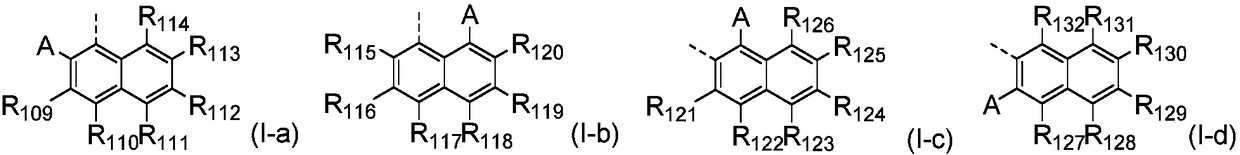Aromatic amine derivative and organic electronic device
A technology of aromatic amine derivatives and atoms, applied in the field of electroluminescence, can solve the problems of low luminous efficiency and poor stability, and achieve the effects of high luminous efficiency and long device life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0246] The present invention will be described below in conjunction with specific embodiment, but the present invention is not limited to following embodiment, it should be understood that appended claims have summarized the scope of the present invention, those skilled in the art should understand under the guidance of the present invention concept It is recognized that certain changes made to the various embodiments of the present invention will be covered by the spirit and scope of the claims of the present invention. Synthesis Example 1: Synthesis of Compound 1
[0247]
[0248] 1-1(8.12g, 25mmol), 1-2(4.52g, 12.55mmol), Pd(dba) 2 (430mg, 0.75mmol) and sodium tert-butoxide (7.23g, 75mmol) were placed in a 500mL dry two-necked flask, and 150mL of anhydrous toluene and 4.3mL of tBu 3 P, stirred overnight at 100°C. After the reaction was cooled, it was washed with water, dried, and purified by column chromatography to obtain Compound 1 (3.5 g, 33.3%) as a yellow solid pr...
Synthetic example 2
[0249] Synthesis Example 2: Synthesis of Compound 2
[0250]
[0251] 2-1(8.5g, 25mmol), 2-2(4.52g, 12.55mmol), Pd(dba) 2 (430mg, 0.75mmol) and sodium tert-butoxide (7.23g, 75mmol) were placed in a 500mL dry two-necked flask, and 150mL anhydrous toluene and 4.3mLtBu 3 P, stirred overnight at 100°C. After the reaction was cooled, it was washed with water, dried, and purified by column chromatography to obtain Compound 2 (3.8 g, 33.5%) as a yellow solid product.
Synthetic example 3
[0252] Synthesis Example 3: Synthesis of Compound 3
[0253]
[0254] 3-1 (9.8g, 30mmol), 3-2 (5.1g, 15.0mmol), Pd (dba) 2 (516mg, 0.9mmol) and sodium tert-butoxide (8.7g, 90mmol) were placed in a 500mL dry two-necked flask, and 150mL of anhydrous toluene and 5.1mL of tBu 3 P, stirred overnight at 100°C. After the reaction was cooled, it was washed with water, dried, and purified by column chromatography to obtain compound 3 (5.2 g, 41%) as a yellow solid product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com