Immobilizing method for micro-molecular ligand containing secondary amine and/or tertiary amine groups
A technology of small molecule ligands and tertiary amine groups, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, organic chemistry, etc., can solve problems such as weak interactions and difficult fixation, and achieve Fixing method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
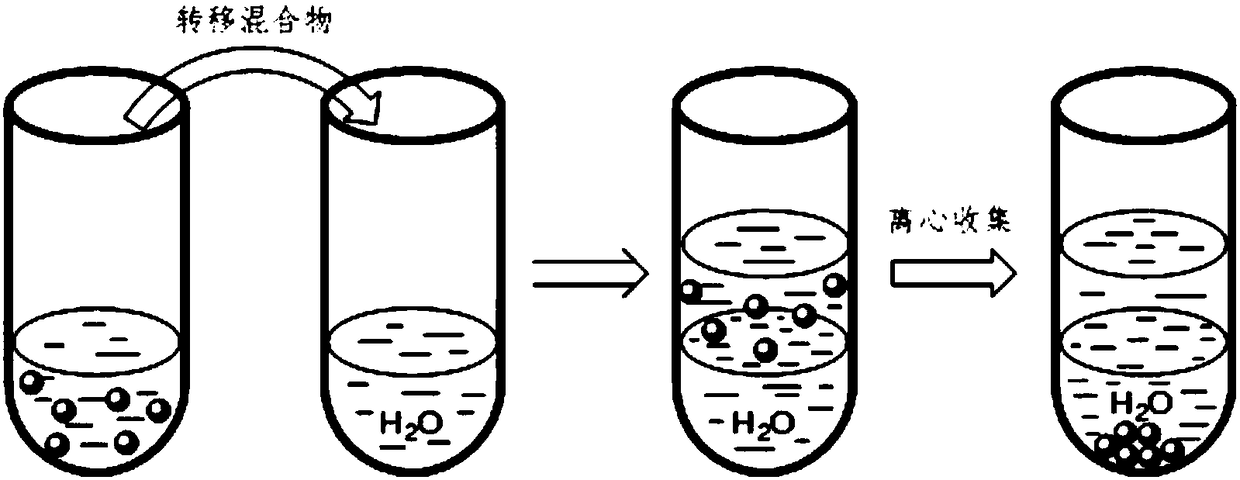
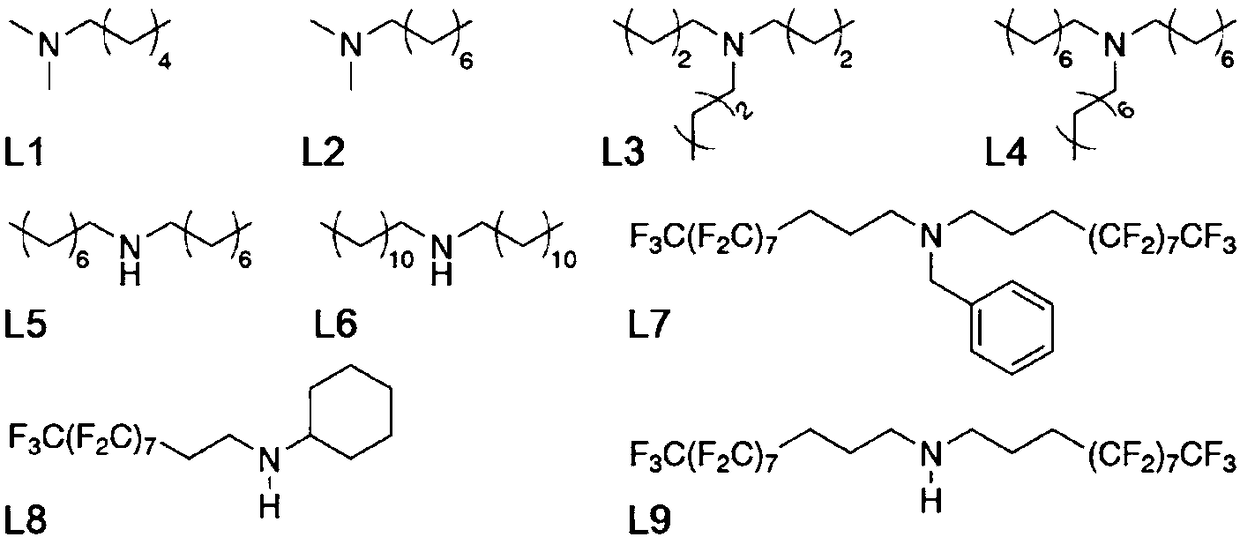
[0025] Weigh 39 mg small molecule ligand L1 (see attached figure 2 ) into a reaction flask filled with 6mL of ethyl acetate, vigorously stirred until completely dissolved, and 300 mg of titanium dioxide-loaded ruthenium catalyst was added to the above reaction flask. After maintaining stirring for 2 hours, the mixture was transferred to a centrifuge tube containing 6 mL of water. Centrifuge at 3000 rpm for 20 minutes, collect the centrifuged solid and wash it with deionized water, and finally vacuum-dry the product at room temperature for 24 hours to obtain an organic-inorganic hybrid catalyst.
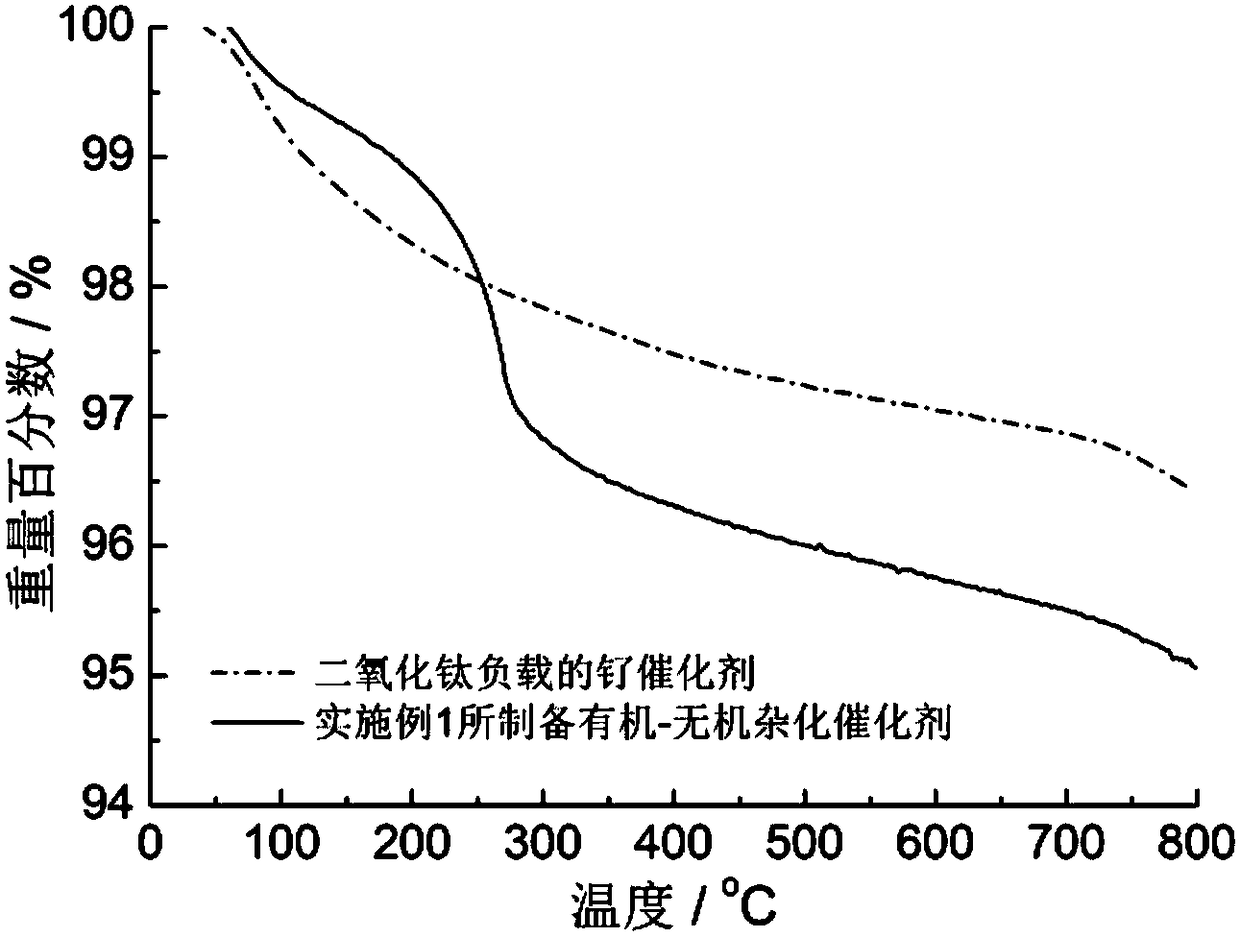
[0026] figure 1 Schematic diagram of the immobilization method for small molecule ligands containing secondary and / or tertiary amine groups. image 3 For the thermogravimetric analysis curve of the organic-inorganic hybrid catalyst prepared in the above examples in air, as the temperature increases, the organic components are continuously oxidized and decomposed, and finally only t...
Embodiment 2
[0028] Weigh 27mg small molecule ligand L2 (see attached figure 2 ) into the reaction flask filled with 6mL ethyl acetate, stirred vigorously until completely dissolved, and added 648mg of titanium dioxide-supported ruthenium catalyst in the above-mentioned reaction flask. After maintaining stirring for 6 hours, the mixture was transferred to a centrifuge tube containing 6 mL of water. Centrifuge at 5000 rpm for 30 minutes, collect the centrifuged solid and wash it with deionized water, and finally vacuum-dry the product at room temperature for 36 hours to obtain an organic-inorganic hybrid catalyst.
[0029] Thermogravimetric analysis showed that the content of the small molecule ligands in the organic-inorganic hybrid catalyst prepared in the above examples was 1.0 wt%.
Embodiment 3
[0031] Weigh 75mg small molecule ligand L3 (see attached figure 2 ) was added to the reaction flask filled with 6mL of n-pentane, vigorously stirred until completely dissolved, and 300mg of titanium dioxide-supported ruthenium catalyst was added in the above-mentioned reaction flask. After maintaining stirring for 2 hours, the mixture was transferred to a centrifuge tube containing 6 mL of water. Centrifuge at 5000 rpm for 30 minutes, collect the centrifuged solid and wash it with deionized water, and finally vacuum-dry the product at room temperature for 24 hours to obtain an organic-inorganic hybrid catalyst.
[0032] Thermogravimetric analysis showed that the content of the small molecule ligand in the organic-inorganic hybrid catalyst prepared in the above example was 1.2 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com