Bimetal oxide and graphene composite material and preparation method thereof
A technology of double metal oxide and composite materials, which is applied in the field of preparation of graphene composite materials, can solve the problems that affect the properties, types and quantities of materials that are difficult to accurately control, and the molar ratio is difficult to accurately control, and achieve the effect of avoiding damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of double metal oxide / graphene composite material preparation method, concrete steps are as follows:
[0039] Step 1, preparing a graphene oxide dispersion with a concentration of 3 mg / mL, adding metal chloride salt to it, controlling the concentration at 1 to 10 mmol / L, and recording it as solution 1; preparing a potassium metal cyanate solution with a concentration equal to that of metal chloride The salt is the same, and is recorded as solution 2;
[0040]Step 2: After stirring solution 1 and solution 2 evenly, add solution 2 dropwise to solution 1 at a volume ratio of 2:3 at a rate of 0.5-1 mL / min, and stir for 10 min;
[0041] Step 3, transfer the above mixed solution to a high temperature and high pressure reactor, and conduct a hydrothermal reaction at 120°C for 6 hours; continue to adjust the temperature to 180-220°C, and conduct a hydrothermal reaction for 18 hours. After natural cooling, the load AB is obtained 2 o 4 graphene hydrogel materials.
Embodiment 2
[0043] The steps of this embodiment are basically the same as those of Example 1, except that in step 1, the metal chloride salt is NiCl 2 , potassium metal cyanate is K 3 [Fe(CN) 6 ]; Step 3 continues to regulate the temperature to 180 ° C.
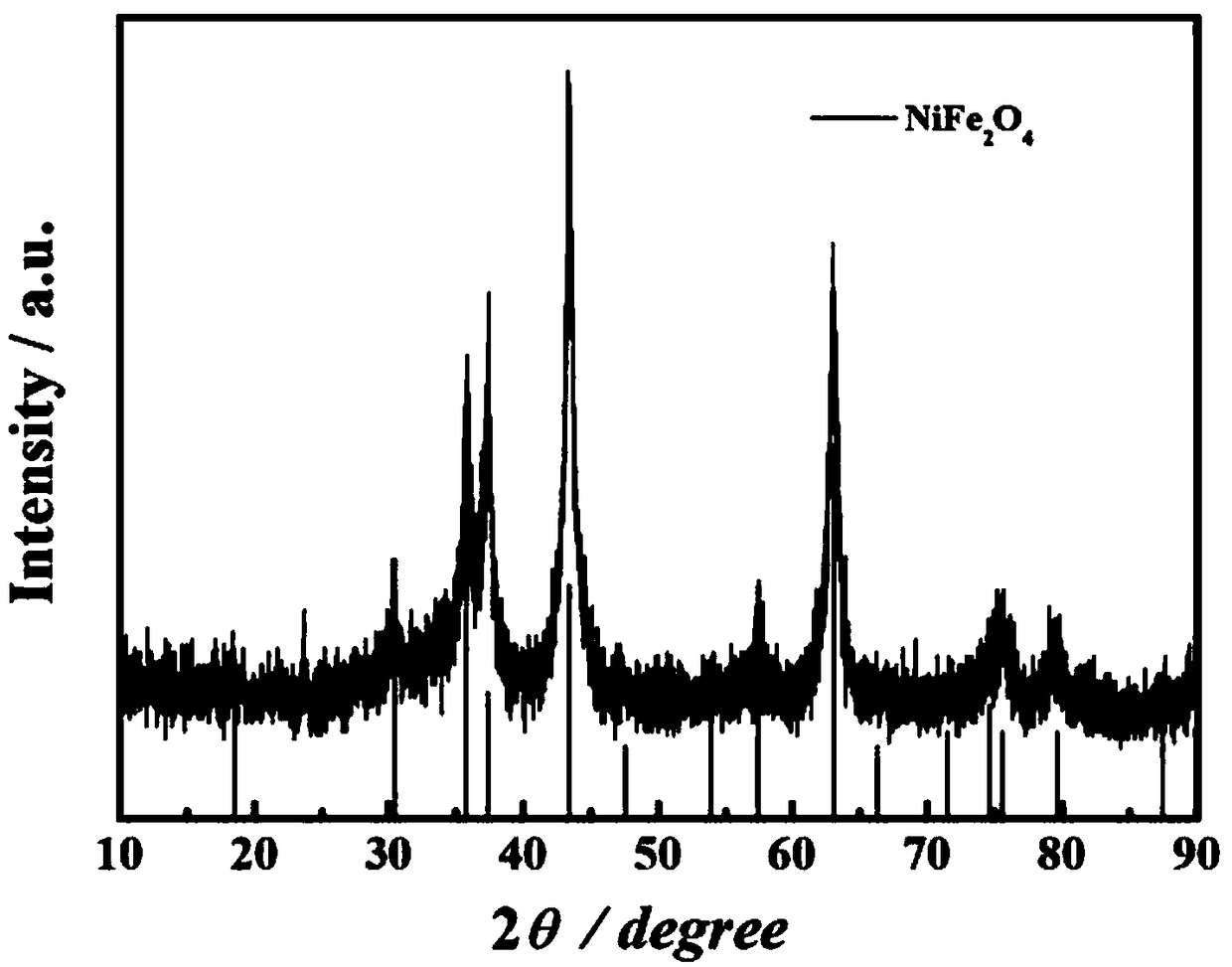
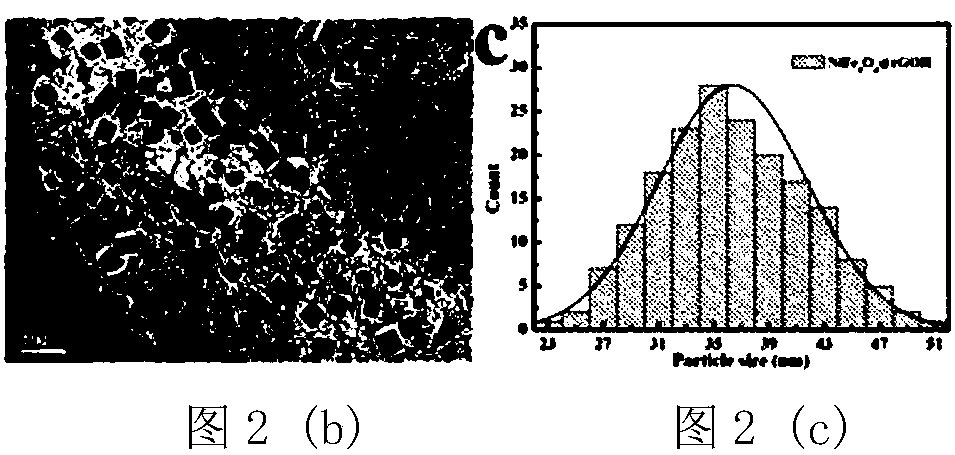
[0044] figure 1 It is the XRD pattern of the obtained material, and it can be seen from the corresponding peak position that the synthesized material is pure phase NiFe 2 o 4 / Graphene. Figure 2(a)-Figure 2(c) For this example NiFe 2 o 4 / Graphene: Figure 2(a) SEM, the magnification of the figure is 30000 times; Figure 2(b) TEM, it can be seen that the obtained NiFe 2 o 4 The nanocubes are loaded on the graphene surface; Figure 2(c) shows the particle size distribution diagram, which shows that the particle size is between 30 and 40nm, and the particle size distribution is relatively uniform.
[0045] Figure 3(a) for NiFe 2 o 4 / graphene material at scanning speeds of 5, 10, 20, 40mV s -1 The cyclic voltammetry curve below,...
Embodiment 3
[0047] The steps of this embodiment are basically the same as those of Embodiment 2, the difference is that in step 1, K 3 [Fe(CN) 6 ] for K 3 [Co(CN) 6 ]; In step 3, "control the temperature to 180°C" is replaced with "control the temperature to 200°C". The synthesized material is NiCo 2 o 4 / graphene material.
[0048] The prepared NiCo 2 o 4 / Graphene material electrochemical performance test shows that the current density is 1, 2, 3, 6A g -1 , the discharge specific capacity is 201, 187, 171, 155F g -1 ; when the current density increases to 6A g -1 , the discharge specific capacitance is still 1A g -1 When discharging 77% of the specific capacitance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com