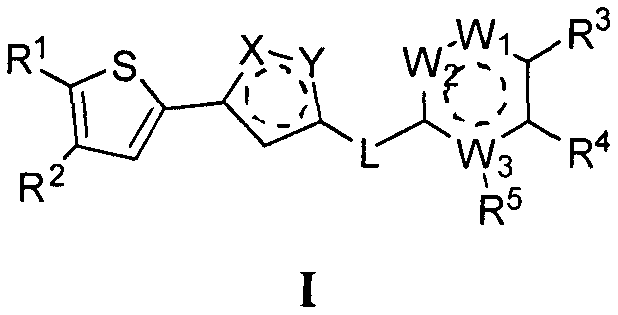
Compounds and use thereof in anti-AML drugs
A compound, independent technology, applied in the field of ITD mutant selective inhibitor of FMS-like tyrosine kinase 3, azole ring substituted thiophene compounds, can solve the problem of expression level as an indicator and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
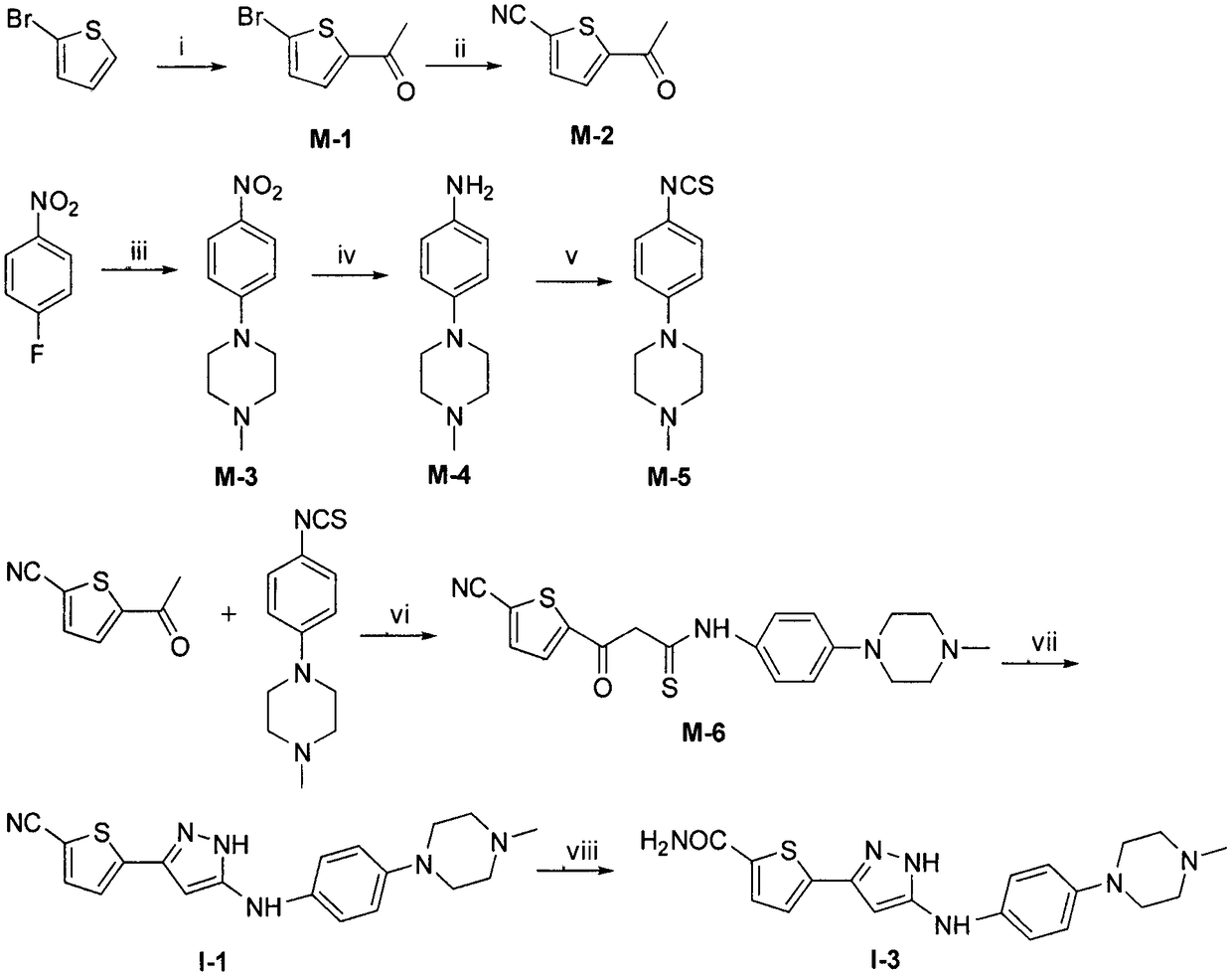
[0192] 2-Acetyl-5-bromothiophene (M-1)
[0193] Add 2-bromothiophene (2.00g, 12.3mmol) and acetic anhydride (2.51g, 24.6mmol) into a 100ml eggplant-shaped bottle, slowly add perchloric acid (3.71g, 36.9mmol) dropwise under ice-bath conditions, add 0.5h After that, remove the ice bath and stir at room temperature. After 6h TLC showed no starting material remained. Add ice water, adjust the pH to neutral with sodium carbonate, extract with ethyl acetate (25ml×3), combine the organic phases, wash twice with saturated brine, anhydrous MgSO 4 dry. The solvent was distilled off under reduced pressure, and the crude product was subjected to column chromatography (PE: EA = 50: 1) to obtain 2.03 g of light yellow oil, with a yield of 80.6%.
[0194] MS (m / z): [M+H] + 204.9, 206.9.
Embodiment 2
[0196] 2-Acetyl-5-cyanothiophene (M-2)
[0197]Add M-1 (2.00g, 9.75mmol), cuprous cyanide (2.62g, 29.3mmol), potassium iodide (0.02g, 0.1mmol) and 6ml of anhydrous DMF into a 50ml pressure tube. The temperature was raised to 150° C. under the protection of argon, and after 12 hours, TLC showed that there was no remaining raw material. The reaction solution was poured into a mixture of ammonia water and crushed ice (1:3), stirred to quench the reaction, filtered with suction, and the solid was dried and extracted with ethyl acetate. The mother liquor was extracted with ethyl acetate (20ml×3), the organic phases were combined, washed twice with saturated brine, anhydrous MgSO 4 After drying and concentration under reduced pressure, the crude product was subjected to column chromatography (PE:EA=50:1) to obtain 0.95 g of a yellow oil with a yield of 64.2%.
[0198] 1 H-NMR (300MHz DMSO-d 6 )δ: 2.62 (3H, s, -COC H 3 ), 8.05 (1H, d, J=4.0Hz, -CH-, Thiophene), 8.08 (1H, d, J=4...
Embodiment 3
[0201] 1-Methyl-4-(4-nitrophenyl)piperidine (M-3)
[0202] Add p-fluoronitrobenzene (2.00g, 14.2mmol), 1-methylpiperazine (1.42g, 14.2mmol), potassium carbonate (1.96g, 14.2mmol) and DMSO 25ml in a 100ml single-necked bottle, react at room temperature, After 6h, TLC showed that no starting material remained. The reaction solution was poured into 200ml of water, a brown solid was precipitated, suction filtered, the solid was dried, the mother liquor was extracted with ethyl acetate (40ml×3), the organic phases were combined, washed twice with saturated brine, and anhydrous MgSO 4 dry. After concentration under reduced pressure, it was combined with the dried solid, and the crude product was subjected to column chromatography (PE:EA=6:1) to obtain 2.73 g of yellow needle-like crystals, with a yield of 87.2%.
[0203] 1 H-NMR (300MHz CDCl 3 )δ: 2.36 (3H, s, C H 3 -N-), 2.57 (4H, t, J=5.1Hz, -N-C H 2 -×2), 3.45 (4H, t, J=5.1Hz, CH 3 -N-C H 2 -×2), 6.82-6.85 (2H, m, Ar-H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com