A kind of polypyrazole containing xanthene structure and preparation method thereof
A technology of polypyrazole and xanthene, which is applied in the field of polymer synthesis, can solve the problems of low polymerization efficiency and achieve the effects of simple preparation method, clear structure and good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of xanthene-containing monomers (taking 9,9-bis(4-ethynylphenyl)xanthene as an example)
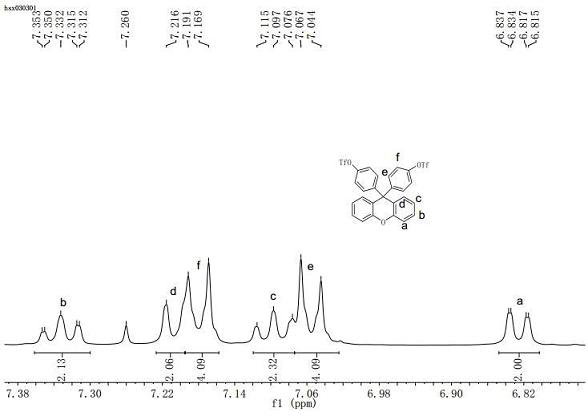
[0040] (1) Add 3.6g (10mmol) of 9,9-bis(4-hydroxyphenyl)phenyl, pyridine (8mL), and dichloromethane (150mL) into a 250mL single-necked bottle, stir and dissolve in an ice bath Finally, drop trifluoromethanesulfonic anhydride (5mL, 30mmol) into the reaction flask dropwise, remove the ice bath after the dropwise addition, return to room temperature and then react for 2h, add 50mL distilled water to quench the reaction, and the organic layer was sequentially Wash with HCl(1N) (2×30mL), water (20mL), and wash the organic phase with anhydrous NaSO 4 Drying, filtration, and rotary evaporation of the solvent gave the crude product, which was recrystallized from methanol and dried to give 9,9-bis(4-(trifluoromethylsulfonyloxy)phenyl)xanthene (5.6 g) as a white solid , the yield was 89%, and the melting point was 112.5-113.5°C. 1 H NMR (400MHz, CDCl 3 ): δ=7.35–7.31(m, 2H), 7...
Embodiment 2
[0044] Preparation of polypyrazole (taking polypyrazole P(1a / 2a) as an example)
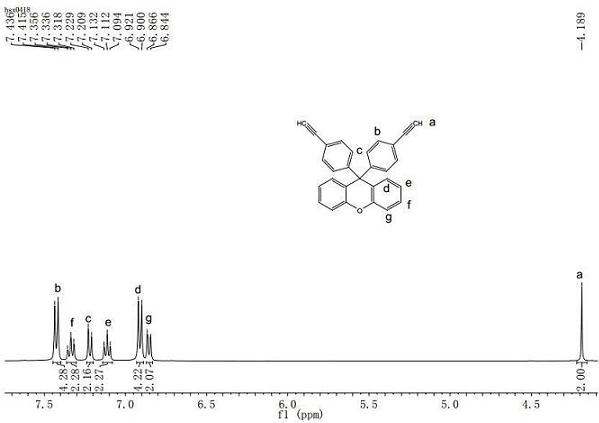
[0045] Add 9,9-bis(4-ethynylphenyl)xanthene (0.3825g, 1mmol), terephthaloyl chloride (0.2030g, 1mmol), Pd(PPh 3 ) 2 Cl 2 (0.0281g, 0.04mmol), CuI (0.0152g, 0.08mmol), after sealing well, back and forth evacuated and filled with nitrogen three times, under the protection of nitrogen balloon, inject freshly steamed tetrahydrofuran (20mL) and triethylamine (0.28mL), at room temperature After reacting for 1h, inject freshly distilled DMF (5mL) and hydrazine hydrate (0.36mL, 3mmol), continue to react at room temperature for 12h, then add the reaction solution dropwise into n-hexane / tetrahydrofuran (v / v=10 / 1) In the mixed solvent, a yellow powder was precipitated, filtered with suction, put the powder into a Soxhlet extractor and extracted it with hot n-hexane for 24 hours, and then dried it in vacuum at 80°C for 12 hours to obtain polypyrazole P(1a / 2a).
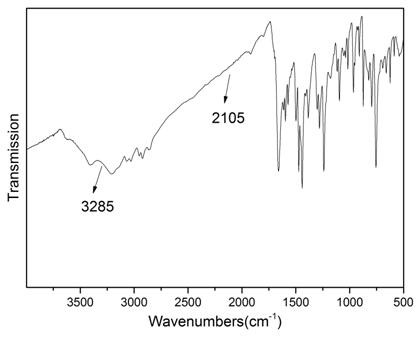
[0046] (1) Structural analysis of polypyraz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com