Preparation method of deuterated methyl parathion
A technology of deuterated methyl parathion and deuterated methanol, applied in the field of chemical analysis, can solve problems such as lack of complete and detailed literature reports, and achieve the effects of good product quality, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
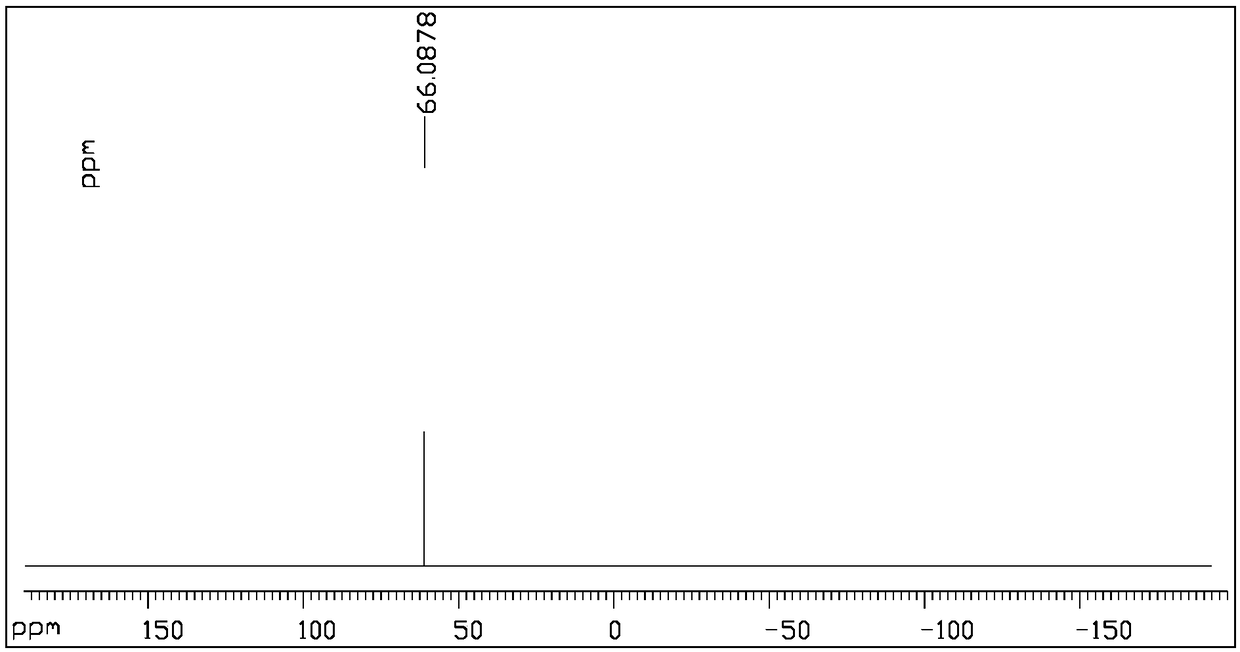
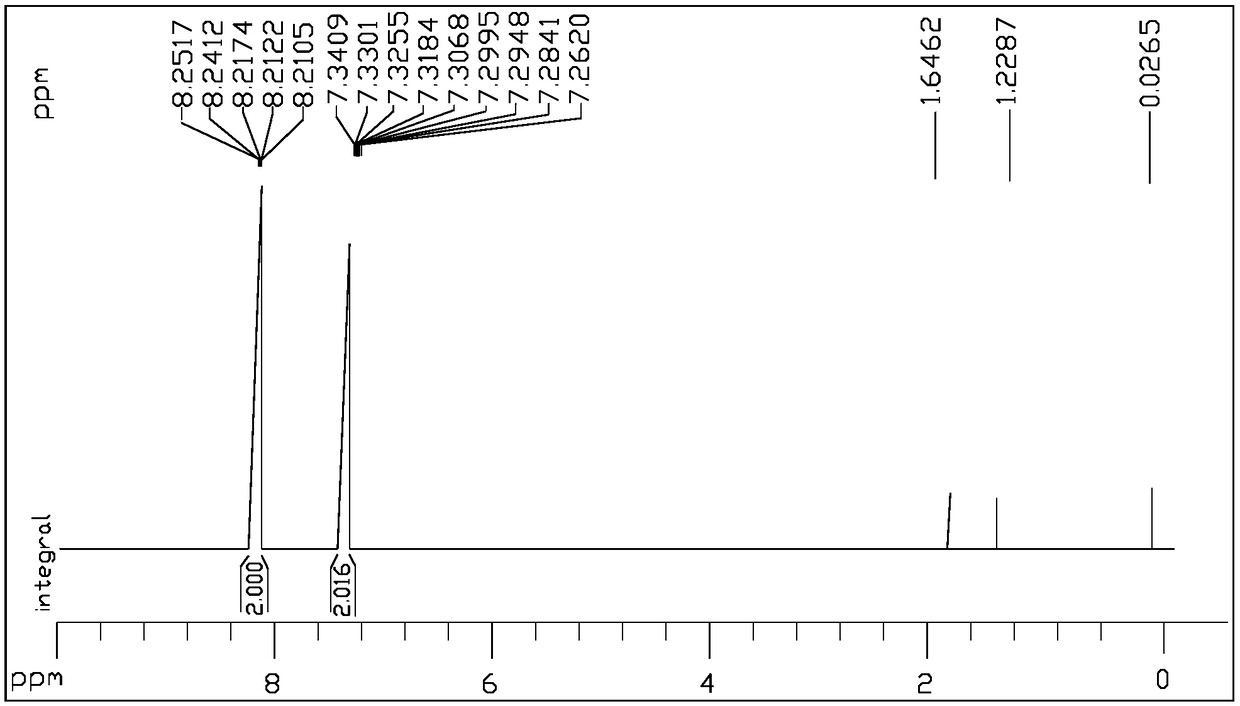
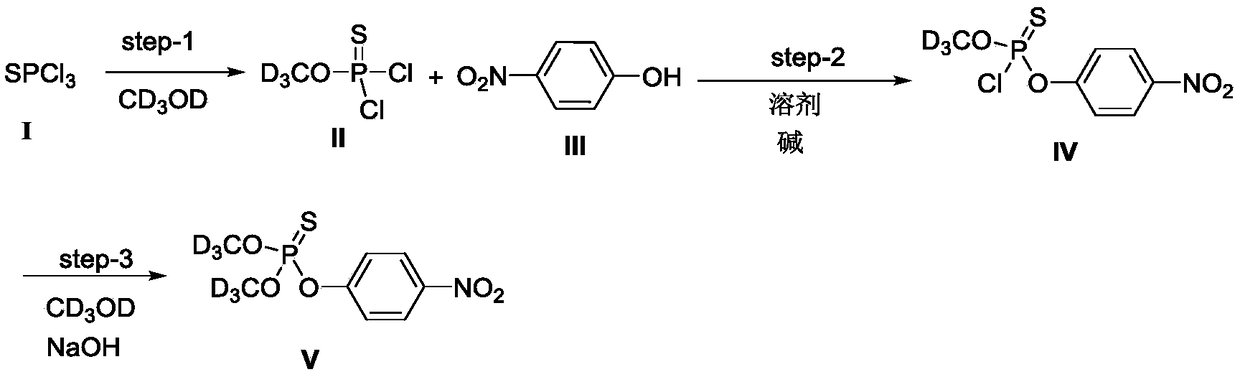
[0040] (1) Phosphor trichloride monoesterifies with deuterated methanol to obtain reaction product II (molecular weight 169.979)
[0041]
[0042] Weigh 20.8g of phosphorus trichloride (compound I, 122.4mmol) into a 100mL round-bottomed flask, cool to -10°C in an ice-salt bath, then add 13.3g of deuterated methanol (367.2mmol) dropwise, in 15-20min After the dropwise addition, raise the temperature to -5°C and continue stirring for 1 hour, add 50 mL of ice water (water at 0°C) to the reaction system, stir for 5 minutes, then separate the liquids, keep the organic phase after liquid separation, and use the water phase after liquid separation Dichloromethane was extracted twice, each with 20 mL of dichloromethane. Combine the organic phase after liquid separation and the organic phase obtained by extraction, dry with 25g of anhydrous magnesium sulfate, filter and concentrate under reduced pressure to obtain reactant II, which is O-(methyl-D3)-thiophosphoryl di chlorine.
[...
Embodiment 2
[0051] (1) Phosphor trichloride monoesterifies with deuterated methanol to obtain reaction product II
[0052]
[0053] Weigh 20.8g of phosphorus trichloride (compound I, 122.4mmol) into a 100mL round-bottomed flask, cool to -10°C in an ice-salt bath, then add 13.3g of deuterated methanol (367.2mmol) dropwise, in 15-20min After the dropwise addition, raise the temperature to -5°C and continue stirring for 1 hour, add 50 mL of ice water (water at 0°C) to the reaction system, stir for 5 minutes, then separate the liquids, keep the organic phase after liquid separation, and use the water phase after liquid separation Extract twice with dichloromethane, each extraction with 20 mL of dichloromethane. Combine the organic phase after liquid separation and the organic phase obtained by extraction, dry with 25g of anhydrous magnesium sulfate, filter and concentrate under reduced pressure to obtain reactant II, which is O-(methyl-D3)-thiophosphoryl di chlorine.
[0054] (2) The rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com