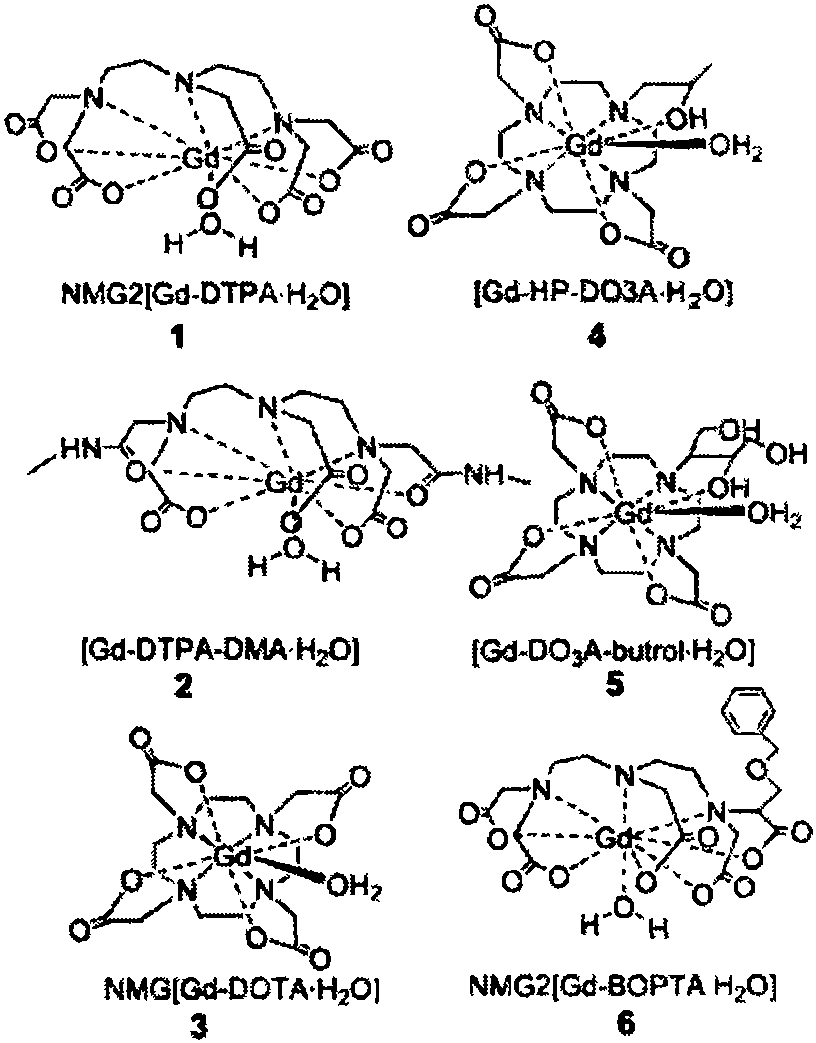
Preparation method for contrast medium intermediate for medical diagnosis
A technology for medical diagnosis and intermediates, applied in the field of chemical drug synthesis, can solve the problems of low yield, high cost, difficulty in synthesizing macrocyclic compounds, etc., and achieve the effects of low cost, stable quality and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
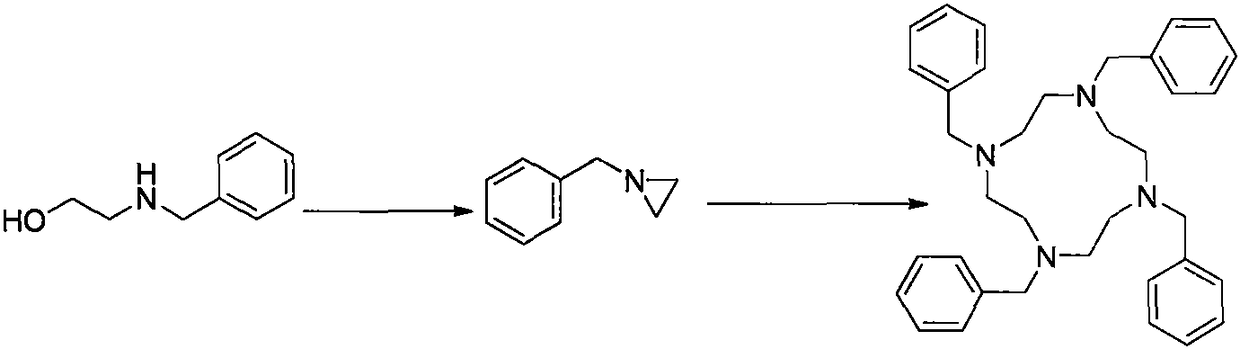
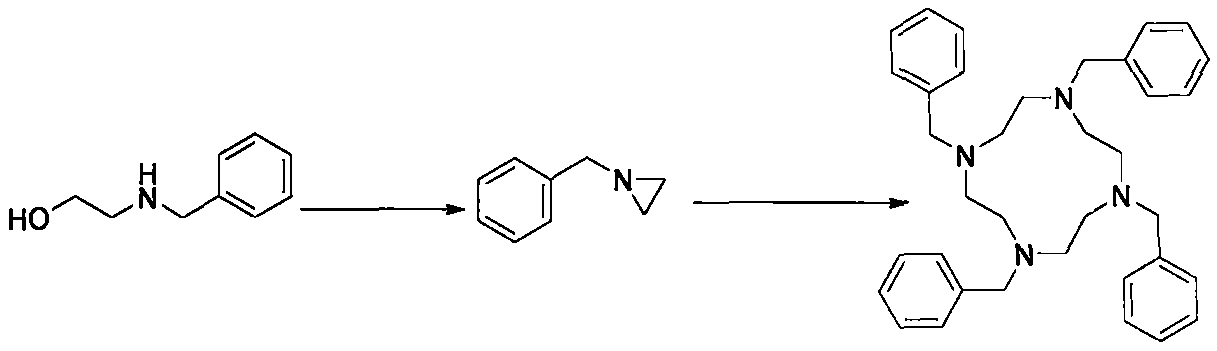
[0026] Put (100.0g, 0.66mol) benzyl ethanolamine and 400g toluene in a 1L three-necked flask, stir evenly, add 100g of concentrated sulfuric acid, and heat up to reflux, heat and reflux for 8 hours, cool to room temperature, add sodium hydroxide Adjust the pH to 10, separate the liquid, and recycle the toluene;
[0027] The resulting aqueous phase was heated to reflux, kept at reflux for 8 hours, cooled to room temperature, and allowed to stand for liquid separation to obtain (66.5 g, 0.50 mol) of an oily intermediate;
[0028] Put the obtained (66.5g, 0.5mol) oil into a reaction flask, add 400g of methanol and 13.3g of concentrated sulfuric acid, heat up to reflux, and keep the temperature for 12 hours. Filter and dry the filter cake to obtain (0.088mol, 47.0g) tetrabenzylcyclene product with a yield of 70.6% and a purity of 99.5%.
Embodiment 2
[0030] Put (100.0g, 0.66mol) benzyl ethanolamine and 500g toluene in a 1L three-neck flask, stir evenly, add 150g of concentrated sulfuric acid, and heat up to reflux, heat and reflux for 12 hours, cool to room temperature, add sodium hydroxide Adjust the pH to 9, separate the liquid, and recycle the toluene;
[0031] The resulting aqueous phase was heated to reflux, kept at reflux for 14 hours, cooled to room temperature, and allowed to stand for liquid separation to obtain (61.8 g, 0.46 mol) of an oily intermediate;
[0032] Put the obtained (61.8g, 0.46mol) oil into a reaction flask, add 800g of methanol and 15.0g of methanesulfonic acid, heat up to reflux, keep the temperature for reaction for 12 hours, after the reaction is completed, cool down to below 0°C, keep warm and crystallize for 8 hours, Suction filtration and drying of the filter cake yielded (0.085 mol, 47.0 g) tetrabenzcyclene product with a yield of 70.6% and a purity of 99.5%.
Embodiment 3
[0034] Put (100.0g, 0.66mol) benzyl ethanolamine and 400g toluene in a 1L three-necked flask, stir evenly, add 100g of concentrated sulfuric acid, and heat up to reflux, heat and reflux for 8 hours, cool to room temperature, add sodium hydroxide Adjust the pH to 8-10, separate the liquid, and recover and reuse the toluene;
[0035] The resulting aqueous phase was heated to reflux, kept at reflux for 8 hours, cooled to room temperature, and allowed to stand for liquid separation to obtain (66.5 g, 0.5 mol) of an oily intermediate;
[0036] Put the obtained (66.5g, 0.5mol) oil into a reaction flask, add 400g of methanol and 20.0g of benzenesulfonic acid, heat up to reflux, and keep the temperature for 16 hours. Suction filtration and drying of the filter cake yielded (0.080 mol, 42.6 g) tetrabenzylcyclene product with a yield of 64.0% and a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com