Trimetazidine hapten and artificial antigen, and preparation methods and applications thereof
A technology of trimetazidine and artificial antigen, which is applied in the biological field and can solve the problems of difficulty in realizing the immunodetection of trimetazidine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
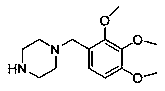
[0047] This example provides a trimetazidine hapten with a structural formula such as formula (I), n=1:
[0048]
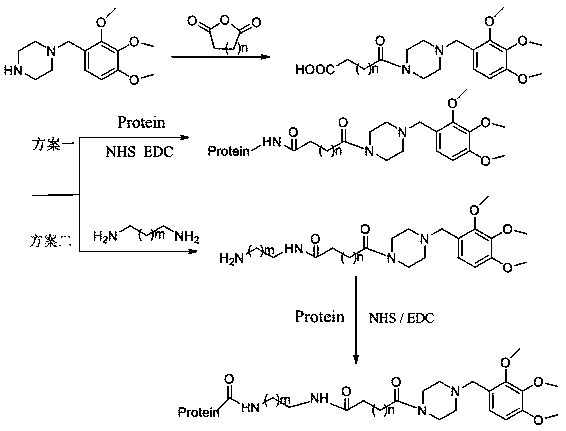
[0049] The preparation method is as follows (such as figure 2 ):
[0050] 1 part of trimetazidine (structural formula such as figure 1 ) was mixed with 10 parts of succinic anhydride, dissolved in 30 parts of 1,4-dioxane, and then 5 drops of triethylamine were added dropwise, and the reaction was carried out under closed stirring at 40°C for 12h. After the reaction, the reaction solution was poured into water, extracted 3 times with 100 parts of ethyl acetate, the extracts were combined, dried with anhydrous Na2SO4, concentrated to obtain a solid, and recrystallized from 75% ethanol to obtain a solid product that was trimetazidine hapten.
[0051]The structure of the obtained product is characterized by: 1H NMR (400MHz, DMSO) δ6.93(d, J=7.6Hz, 1H), 6.50(d, J=7.3Hz, 1H), 3.98(s, J=4.1Hz, 3H ), 3.80(s, J=4.2Hz, 3H), 3.69(s, J=4.1Hz2H), 3.62(s, J=4.4Hz, 3H), ...
Embodiment 2
[0061] This embodiment provides a trimetazidine hapten, the structural formula is as formula (I), and n is 2.
[0062] The preparation method is as follows:
[0063] After mixing 1 part of trimetazidine with 10 parts of glutaric anhydride, the subsequent steps are the same as in Example 1.
[0064] The structure of the obtained product is characterized by: 1H NMR (400MHz, DMSO) δ6.96(d, J=7.8Hz, 1H), 6.45(d, J=8.1Hz, 1H), 3.94(s, J=4.4Hz, 3H ),3.85(s,J=4.3Hz,3H),3.76(s,J=4.1Hz,2H),3.65(s,J=4.4Hz,3H),3.40(t,J=3.0Hz,4H), 2.42(t, J=2.8Hz, 4H), 2.37(t, J=3.5Hz, 2H), 2.30(t, J=3.6Hz, 2H), 2.02(m, J=4.7Hz, 2H).
[0065] The corresponding artificial antigen can be prepared by using the hapten, and the preparation method is consistent with Example 1.
Embodiment 3
[0067] This embodiment provides a trimetazidine hapten, the structural formula is as formula (I), and n is 3.
[0068] The preparation method is as follows:
[0069] After mixing 1 part of trimetazidine with 10 parts of adipic anhydride, the subsequent steps are the same as in Example 1.
[0070] The structure of the obtained product is characterized by: 1H NMR (400MHz, DMSO) δ6.90(d, J=8.6Hz, 1H), 6.52(d, J=7.8Hz, 1H), 3.87(s, J=5.0Hz, 3H ), 3.76(s, J=4.2Hz, 3H), 3.69(s, J=4.1Hz, 2H), 3.64(s, J=4.4Hz, 3H), 3.43(t, J=3.1Hz, 4H) ,2.50(t,J=3.3Hz,4H),2.31(t,J=3.5Hz,2H),2.18(t,J=3.4Hz,2H),1.54(m,2.7Hz,2H),1.52(m ,.J=4.9Hz, 2H).
[0071] The corresponding artificial antigen can be prepared by using the hapten, and the preparation method is consistent with Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
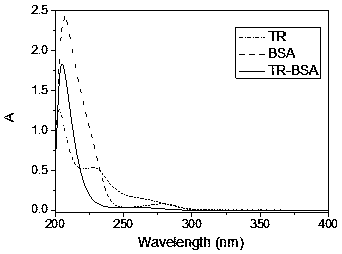
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com