Methods to modulate hepatitis E virus assembly and capsid protein orf2 stability
A virus and protein technology, applied in the fields of molecular biology and virology, can solve problems such as unknown host regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] Example 1: HEV capsid protein ORF2 is acetylated at the conserved amino acid residue K411.
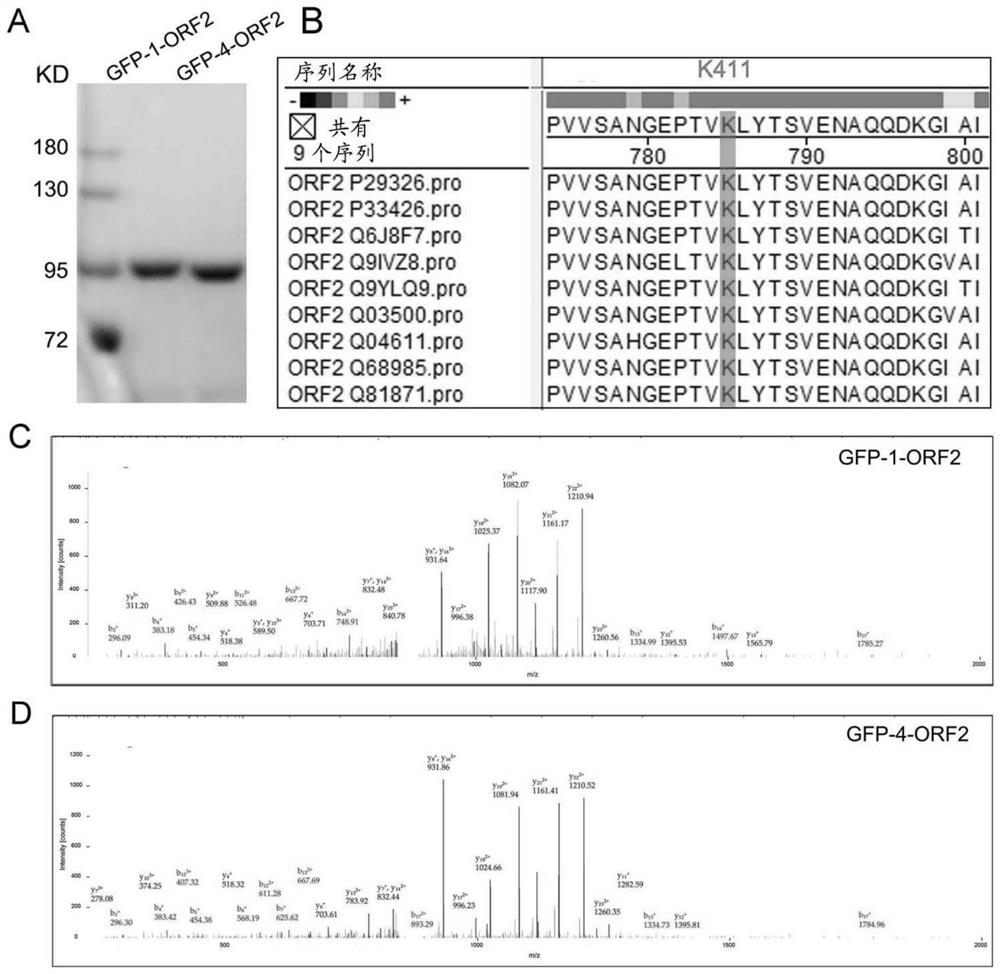
[0224] The N-terminus of ORF2 is not required for its antigenicity and viral particle assembly (39). ORF2 (112-660) shares the same biological properties as the wild-type virus (40). Therefore, using this truncated ORF2, the interaction of HEV ORF2 with host proteins was studied and multiple ORF2-interacting molecules were successfully identified (12). To test whether ORF2 undergoes post-translational modifications, including acetylation, GFP-tagged genotype 1 and 4 ORF2 truncated fragments (GFP-1-ORF2(112-660) and GFP-4-ORF2(112-660)) Introduced into 293T cells. Then, ORF2 was immunoprecipitated using GFP antibody and subjected to acetylation mass spectrometry ( figure 1 A, 1C, 1D). The mass spectrometry results showed that GFP-1-ORF2 and GFP-4-ORF2 in the same peptide EPTVK 411 Acetylated at position K411 of LYTSVEN, this peptide is a highly conserved sequence among 9 exa...
Embodiment 2
[0226] Example 2: Formation of HEV inclusion bodies is dependent on K411 acetylation of ORF2
[0227] Since lysine acetylation plays an important role in the life cycle of the virus, it was decided to evaluate the function of K411 acetylation of HEV ORF2. With wild-type 1-ORF2(112-660), 4-ORF2(112-660), mutant 1-ORF2 fused with GFP tag K411R (112-660), or 4-ORF2 K411R (112-660), HeLa cells were transfected. Cells were fixed, immunofluorescently stained with anti-GFP and anti-tubulin antibodies, and DNA was stained by DAPI. Fluorescence microscopy determined the localization of ORF2 in cells. Surprisingly, GFP-1-ORF2 K411R (112-660) and GFP-4-ORF2 K411R (112-660) were mainly uniformly dispersed in the cytoplasm, whereas both genotype 1 and genotype 4 wild-type GFP-ORF2 (112-660) showed strong inclusion body formation ( figure 2 A, 2B). To test whether the enhanced inclusion body formation is due to N-terminal truncation, full-length ORF2 (ORF2(1-660)) was tested, yieldi...
Embodiment 3
[0230] Example 3: Dynamic assessment of the effect of mutation K411R on inclusion body formation
[0231] It appears that acetylation of HEV ORF2 is involved in regulating inclusion body formation ( figure 2 ). However, the process of inclusion body formation of HEV ORF2 remains unknown. To determine the effect of K411 acetylation on this process, ORF2 inclusion body formation was analyzed by live-cell imaging of wild-type ORF2 and acetylation-depleted ORF2 in HeLa cells. Live cell imaging started 12 hours after GFP-1-ORF2(112-660) transfection and continued for up to 8 hours. Cells had few detectable inclusions at the start of imaging and a few detectable inclusions formed after approximately 30-90 min ( image 3 A). After that, over time, the assembly yielded stronger and stronger bulky inclusion bodies ( image 3 A). These results suggest that acetylation of K411 greatly affects the self-assembly of ORF2 inclusion bodies. To test whether acetylation is one of the de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com