Preparation method of Ni2P-supported Ni-based catalyst, obtained catalyst and application thereof
A catalyst and reaction technology, used in electrolysis components, electrodes, electrolysis processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] One aspect of the present invention provides a Ni 2 The preparation method of P loaded Ni-based catalyst, wherein, the Ni 2 P-supported Ni-based catalysts include nickel foam (NF) support and Ni supported on it 2 P, the method comprising the steps of:
[0050] Step 1. Prepare Ni precursor;
[0051] Step 2, put the Ni precursor and red phosphorus prepared in step 1 into a tube furnace, and perform plasma treatment to obtain the Ni 2 P supported Ni-based catalyst.
[0052] Among them, the preparation method uses plasma treatment to realize the low-temperature preparation of Ni from red phosphorus. 2 P catalyst. Among them, red phosphorus does not need to be processed in any way, for example, it does not need to be processed into nano red phosphorus.
[0053] According to a preferred embodiment of the present invention, in step 1, the Ni precursor is selected from nickel foam (NF).
[0054] In a further preferred embodiment, in step 1, the Ni precursor is selected f...
Embodiment approach
[0057] According to a preferred embodiment of the present invention, the nickel foam (Ni(OH) 2 / NF) is obtained as follows:
[0058] Step 1-1, Ni(NO 3 ) 2 ·6H 2 O, NH 4 F and (NH 2 ) 2 CO is mixed, then water is added;
[0059] Step 1-2, transfer the system of step 1-1 into an autoclave reactor, then add nickel foam (NF), and keep warm for reaction;
[0060] Step 1-3, cool to room temperature after the reaction finishes, collect the foamed nickel in the autoclave reactor and clean and dry it, obtain the foamed nickel (Ni(OH) 2 / NF).
[0061] Among them, Ni(OH) 2 The preparation of / NF is not limited, and in the present invention, the above-mentioned hydrothermal method is preferably used for preparation. Specifically, Ni(NO 3 ) 2 ·6H 2 O is the nickel source, (NH 2 ) 2 CO is a hydroxide corrosion inhibitor and precipitant, which is conducive to the formation of "flower-like" structure Ni(OH) 2 / NF formation. NH 4 F improves Ni(OH) 2 On the surface of the nic...
Embodiment 1
[0111] The Ni foam was first cleaned with 6M HCl, water and ethanol under ultrasonic waves, and then dried under vacuum at 60 °C; the Ni(NO 3 ) 2 ·6H 2 O (1mmol), NH 4 F (4mmol) and (NH 2 ) 2 CO (12 mmol) was added to 20 mL of deionized water; the solution was transferred to a Teflon-lined autoclave reactor (25 mL); then a piece of clean Ni foam (1 cm × 2 cm) with metallic luster was immersed in the solution and placed on the inner wall of the Teflon liner; then the autoclave was sealed and heated to 120°C and kept at this temperature for 16 hours, after cooling to room temperature, the nickel foam was collected and rinsed thoroughly with deionized water and ethanol, and kept Dry in vacuum to get green Ni(OH) 2 / NF.
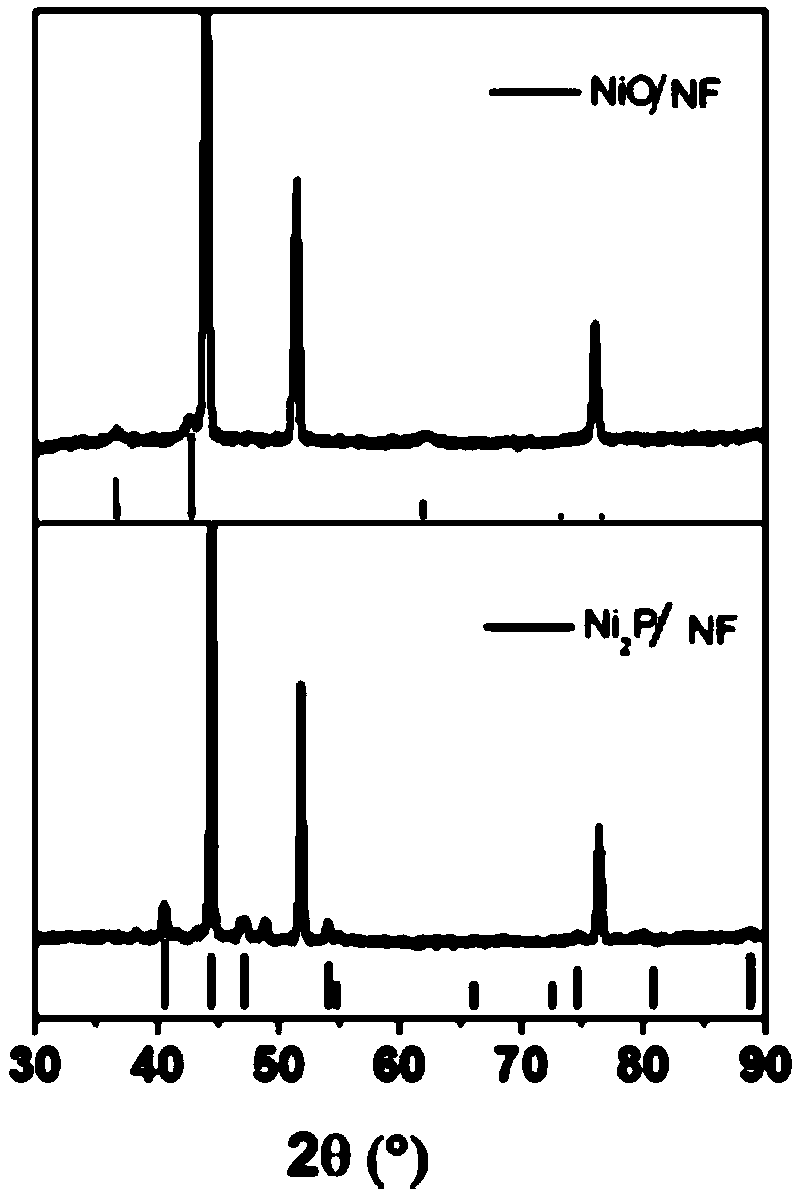
[0112] Ni(OH) 2 / NF was heat-treated at 400 °C for 30 min with a heating rate of 5 °C / min to obtain wheat-colored NiO / NF.
[0113] The NiO / NF was placed in the middle of the tube furnace, and 0.1 g of red phosphorus was placed on the upstream side of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com