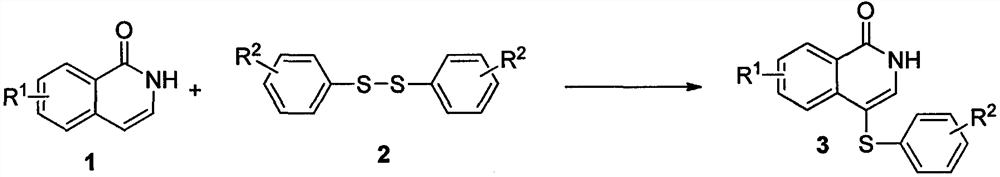
A kind of preparation method of 4-substituted phenylthioisoquinolin-1(2h)-one compound
A technology of phenylthioisoquinoline and ketone compounds, which is applied in the field of organic synthetic chemistry, can solve problems such as harsh reaction conditions, long reaction routes, and poor atom economy, and achieve easy-to-obtain raw materials, mild conditions, and high atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
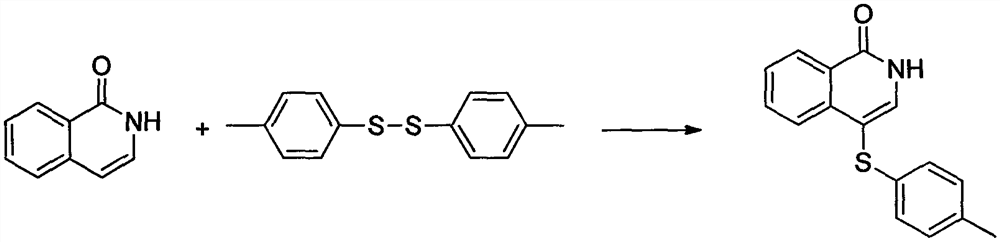
Embodiment 1
[0017]
[0018] At room temperature, add isoquinolin-1(2H)-one (10mmol), bis(4-methylphenyl) disulfide (15mmol), silver hexafluoroantimonate (7mmol) and Dichloroethane (6 mL). The reaction mixture was then reacted at 80°C for 12 hours. Stop reaction, concentrate under reduced pressure to obtain crude product, wash with the mixed eluent of sherwood oil and ethyl acetate at last, flash column chromatography obtains corresponding product 4-(4-methylphenylsulfanyl) isoquinoline-1 ( 2H)-ketone. Yield 96%; white solid, melting point 165-167°C; 1 H NMR (400MHz, CDCl 3 )δ11.74(s, 1H), 8.41(d, J=8.0Hz, 1H), 7.96(d, J=8.1Hz, 1H), 7.71(s, 1H), 7.62(t, J=7.5Hz, 1H), 7.41(t, J=7.6Hz, 1H), 7.03(dd, J=20.6, 8.1Hz, 4H), 2.26(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ164.11, 138.31, 135.79, 135.25, 133.38, 130.81, 129.83, 127.83, 127.40, 127.11, 126.11, 125.51, 109.06, 20.91.
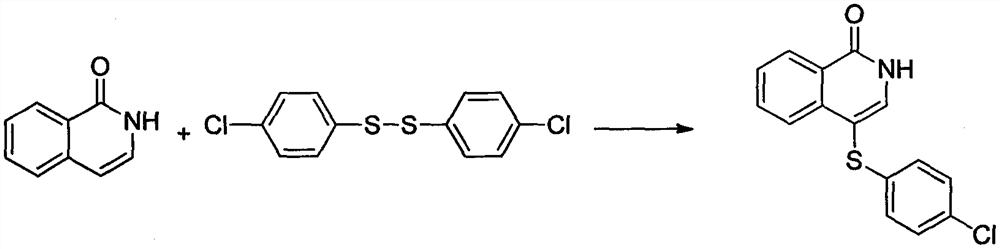
Embodiment 2
[0020]
[0021] At room temperature, sequentially add isoquinolin-1(2H)-one (10mmol), bis(4-chlorophenyl) disulfide (11mmol), silver hexafluoroantimonate (8mmol) and di Ethyl chloride (6 mL). The reaction mixture was then reacted at 80°C for 11 hours. Stop reaction, concentrate under reduced pressure to obtain crude product, wash with the mixed eluent of sherwood oil and ethyl acetate at last, flash column chromatography obtains corresponding product 4-(4-chlorophenylsulfanyl) isoquinoline-1 (2H )-ketone. Yield 90%; white solid, melting point 158-160°C; 1 H NMR (400MHz, CDCl 3 )δ11.36(s, 1H), 8.46(d, J=7.9Hz, 1H), 7.91(d, J=8.0Hz, 1H), 7.68(d, J=9.0Hz, 2H), 7.56(t, J=7.5Hz, 1H), 7.17(d, J=8.1Hz, 2H), 7.06(d, J=8.1Hz, 2H); 13 C NMR (101MHz, CDCl 3 )δ164.01, 137.89, 135.90, 135.84, 133.56, 131.64, 129.17, 127.97, 127.70, 126.44, 125.38, 107.47, 100.00.
Embodiment 3
[0023]
[0024] At room temperature, sequentially add isoquinolin-1(2H)-one (10mmol), bis(2-fluorophenyl) disulfide (10mmol), silver hexafluoroantimonate (11mmol) and di Ethyl chloride (6 mL). The reaction mixture was then reacted at 100°C for 9 hours. Stop reaction, concentrate under reduced pressure to obtain crude product, wash with the mixed eluent of sherwood oil and ethyl acetate at last, flash column chromatography obtains corresponding product 4-(2-fluorophenylsulfanyl) isoquinoline-1 (2H )-ketone. Yield 90%; light yellow solid, melting point 175-177°C; 1 H NMR (400MHz, CDCl 3 )δ11.81(s, 1H), 8.46(d, J=9.0Hz, 1H), 8.00(d, J=8.0Hz, 1H), 7.75(s, 1H), 7.71(ddd, J=8.3, 7.3 , 1.4Hz, 1H), 7.59-7.53(m, 1H), 7.19-7.03(m, 2H), 6.95-6.84(m, 2H); 13 C NMR (101MHz, CDCl 3 )δ164.39, 159.49 (d, J C-F =244.8Hz), 138.15, 136.36, 133.57, 128.71 (d, J C-F =1.5Hz), 127.88, 127.66, 127.44 (d, J C-F =7.5Hz), 126.32, 125.34, 124.63 (d, J C-F =3.3Hz), 115.71, 115.50, 106.31.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com