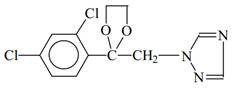
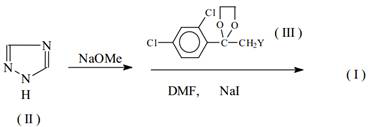
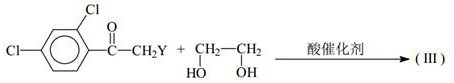
A kind of synthetic method of penconazole intermediate
A synthesis method and intermediate technology, applied in the synthesis field of penconazole intermediates, can solve the problems of high price and difficult operation, and achieve the effects of convenient operation, no corrosion of equipment, and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0103] The present invention is carried out like this, a kind of synthetic method of penconazole intermediate, mainly is the synthetic method of 2,4-dichloroacetophenone, 2-bromo-1-(2,4-dichlorophenyl) Synthetic method of ethyl ketone and synthetic method of ketal.
[0104] 2, the synthetic method of 4-dichloroacetophenone, concrete steps are:
[0105] (1) In a 250ml three-necked bottle, install a stirrer at the middle port, install a dropping funnel and a condenser tube at the two ports, install a calcium chloride drying tube at the upper end of the condenser tube, and connect a hydrogen chloride gas absorption device;
[0106] (2) Quickly weigh 20g (0.15 mol) of anhydrous aluminum trichloride powder, put it into a three-necked bottle, then add 30 ml m-dichlorobenzene, add 6 ml (0.06 mol) acetic anhydride dropwise under stirring, about 20 minutes to finish dripping;
[0107] (3) Then keep boiling slightly on the heating mantle for half an hour until no hydrogen chloride gas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com