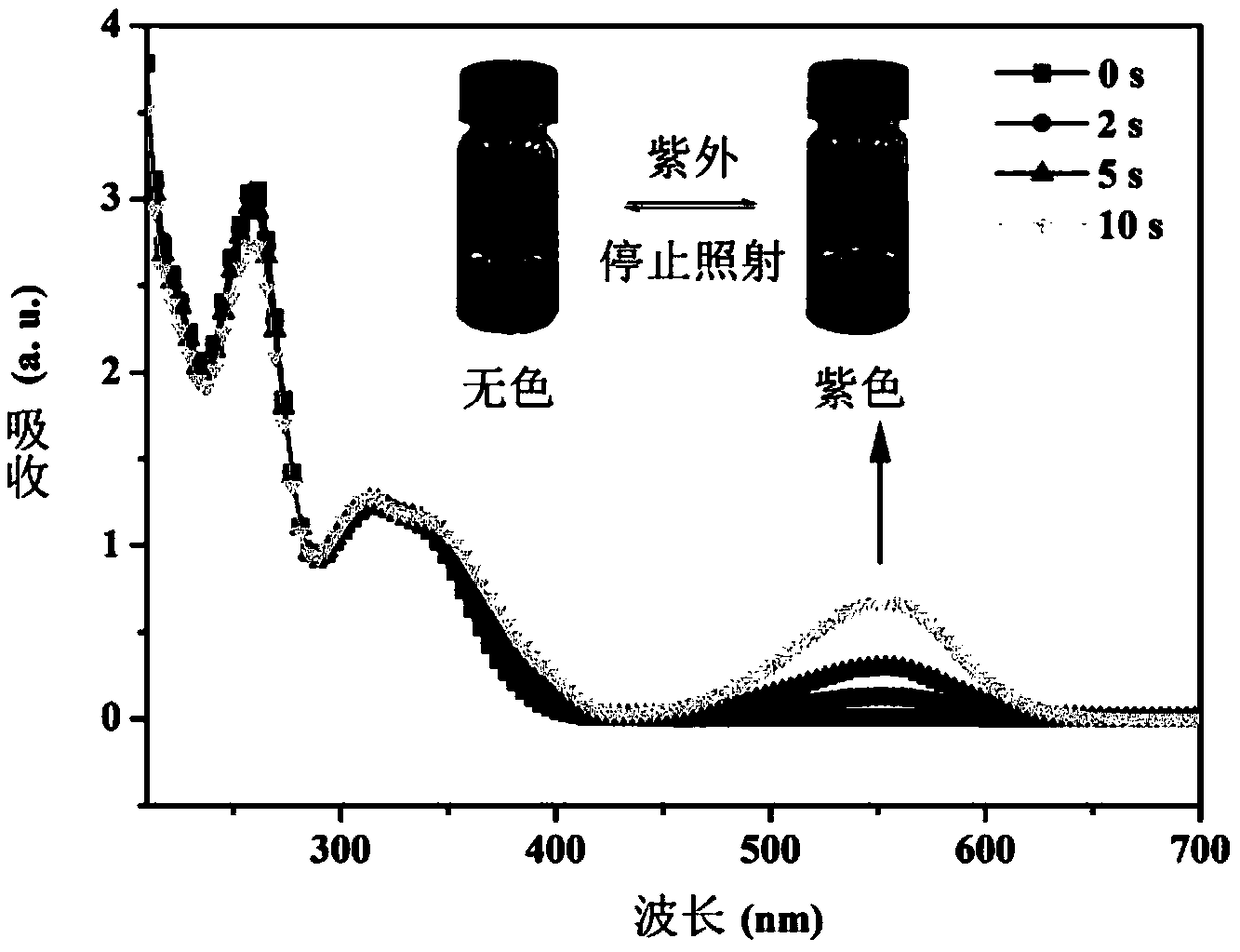
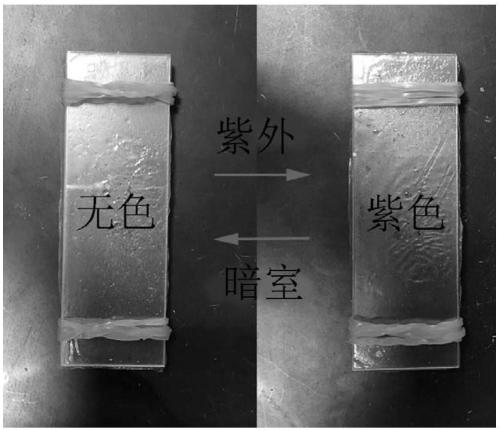
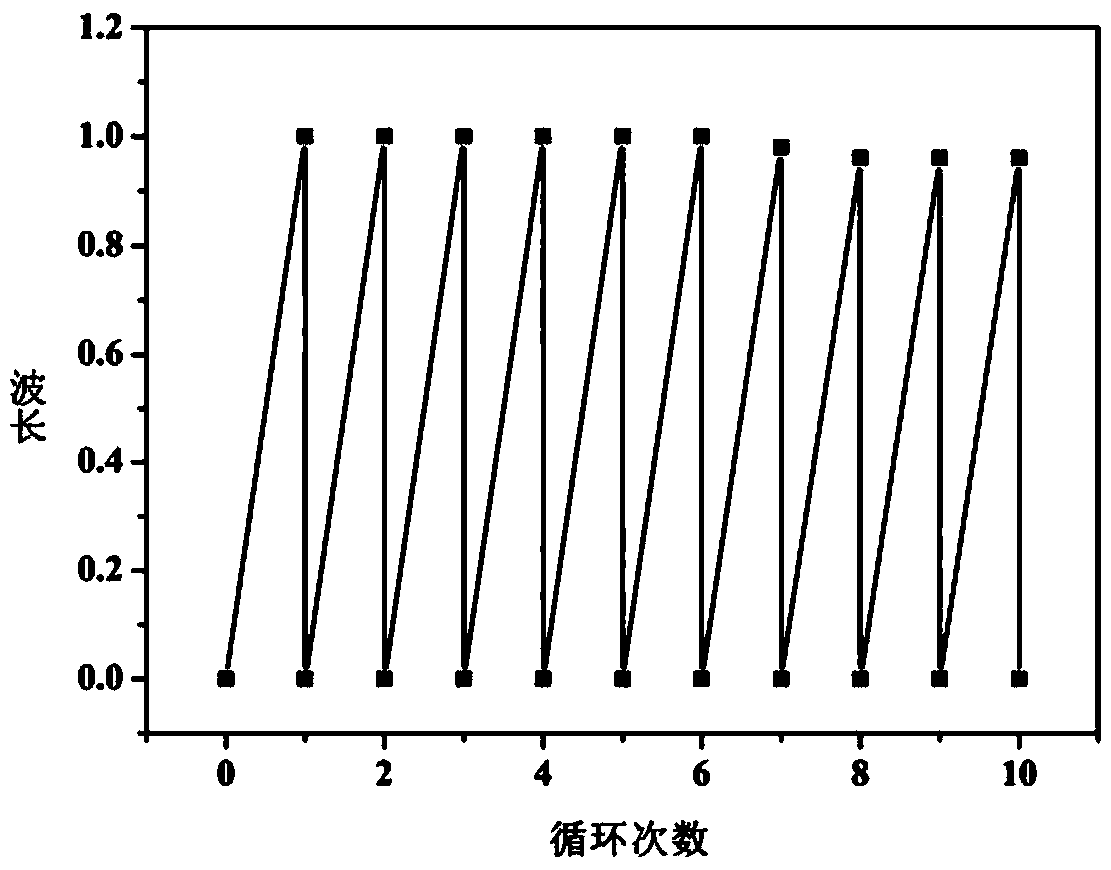
Synthesis of indolin spiropyran derivative with photochromic property and ion detection function
An indoline spiropyran derivative and ion detection technology are applied in the field of indoline spiropyran derivatives, and can solve the problems of inability to store information for a long time, poor thermophotochemical stability, short discoloration recovery time, etc., and achieve post-processing. Simple, productive, and compatible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Preparation of 5'-bromo-1'-ethyl-3',3'-dimethyl-6-nitrospiro[benzopyran-2,2'-indoline]
[0025] The first step, the synthesis of 5-bromo-2,3,3-trimethyl-3H-indole
[0026]
[0027] 6.5g (29.5mmol) of 4-bromophenylhydrazine hydrochloride and 2.5g (29.5mmol) of 3-methyl-2-butanone were added to a 100mL three-necked flask, and under magnetic stirring, 50mL of acetic acid was added, Reflux reaction for 8h. After the reaction was completed, the acetic acid was distilled off, and the remaining liquid was treated with saturated NaHCO 3 The pH of the solution was adjusted to around 9. Partially extracted with ethyl acetate, the obtained organic phase was washed with anhydrous MgSO 4 Dry, filter off MgSO 4 And spin to dry the solvent to obtain 5-bromo-2,3,3-trimethyl-3H-indole crude product;
[0028] The second step, the synthesis of 5-bromo-1-ethyl-2,3,3-trimethyl-3H-indolium bromide
[0029]
[0030] 3.4g (14.4mmol) of 2,3,3-trimethylindole, 1.6g (14.9mmol) of ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com