Preparation method of tirofiban hydrochloride
A technology for tirofiban and hydrochloric acid, which is applied in the field of preparation of tirofiban hydrochloride and N--O-[4-butyl]-L-tyrosine hydrochloride monohydrate, which can solve tedious operations and other problems, to achieve the effect of simple process, mild and controllable reaction, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention shows a kind of preparation method of tirofiban hydrochloride, it comprises the following steps:
[0035] S1. Dissolve N-(n-butylsulfonyl)-O-[4-(4-piperidinyl)butyl]-L-tyrosine represented by compound (I) in dilute hydrochloric acid solution, add activated carbon Thoroughly stir to decolorize, filter;
[0036] S2, the filtrate prepared in step S1 is extracted and washed with ethyl acetate, and the ethyl acetate phase is separated;
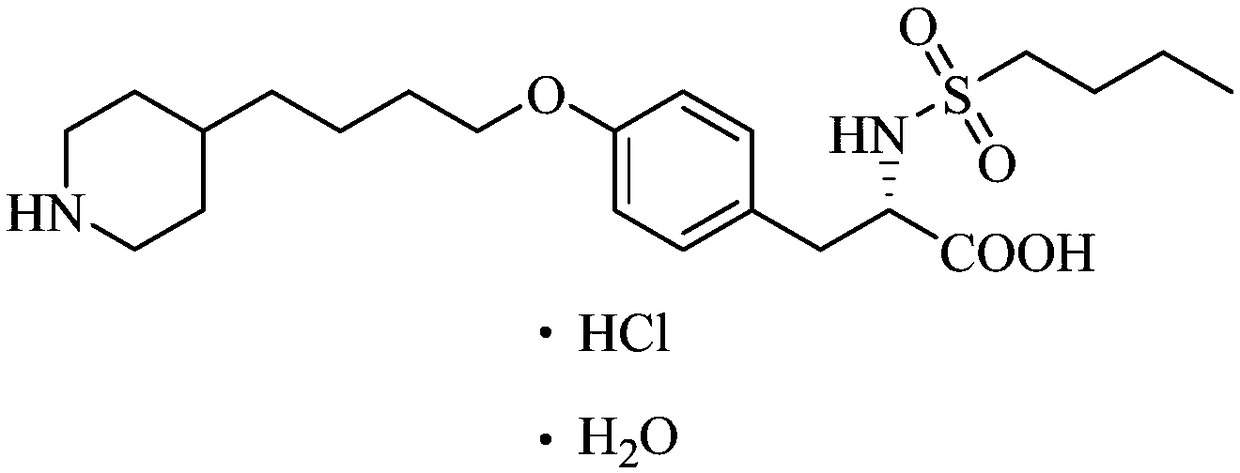
[0037] S3, the product solution that step S2 is made is added dropwise in the hydrochloric acid solution and is stirred and crystallized, obtains the tirofiban hydrochloride shown in white solid namely compound (II); The chemical equation of described reaction is as follows:
[0038]
Embodiment 1
[0041] This embodiment shows the first specific implementation of a preparation method of tirofiban hydrochloride, which comprises the following steps:
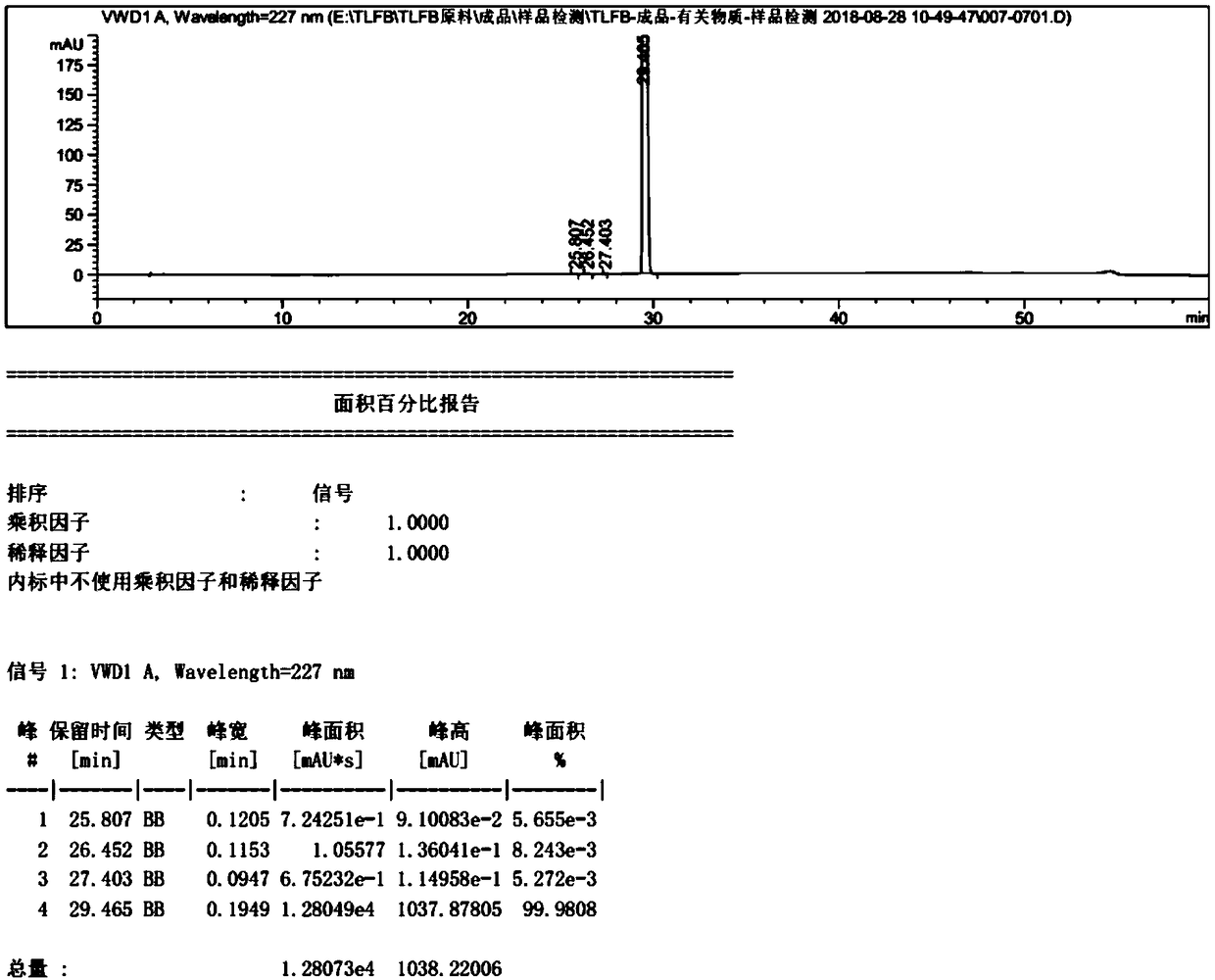
[0042] Compound (I) N-(n-butylsulfonyl)-O-[4-(4-piperidinyl)butyl]-L-tyrosine (25g, 0.057mol) described in step S1 was added to 375ml , in 0.3mol / L dilute hydrochloric acid solution, raise the temperature to 25°C, and fully stir until it dissolves. Add 2.5g of activated carbon, raise the temperature to 40°C, stir thoroughly for 30min, and filter. The filtrate kept at 40°C was extracted and washed with 250 ml of ethyl acetate for 30 min, and the ethyl acetate phase was separated. After maintaining the temperature of the prepared product solution at 25°C, drop it into 375ml, 2mol / L hydrochloric acid solution, stir and crystallize for 3h, and then filter and dry. 21.68 g of white solid was obtained, the yield was 77.1%, and the HPLC purity was 99.98%.
[0043] In this embodiment, the method for HPLC detection of tirofiban hyd...
Embodiment 2
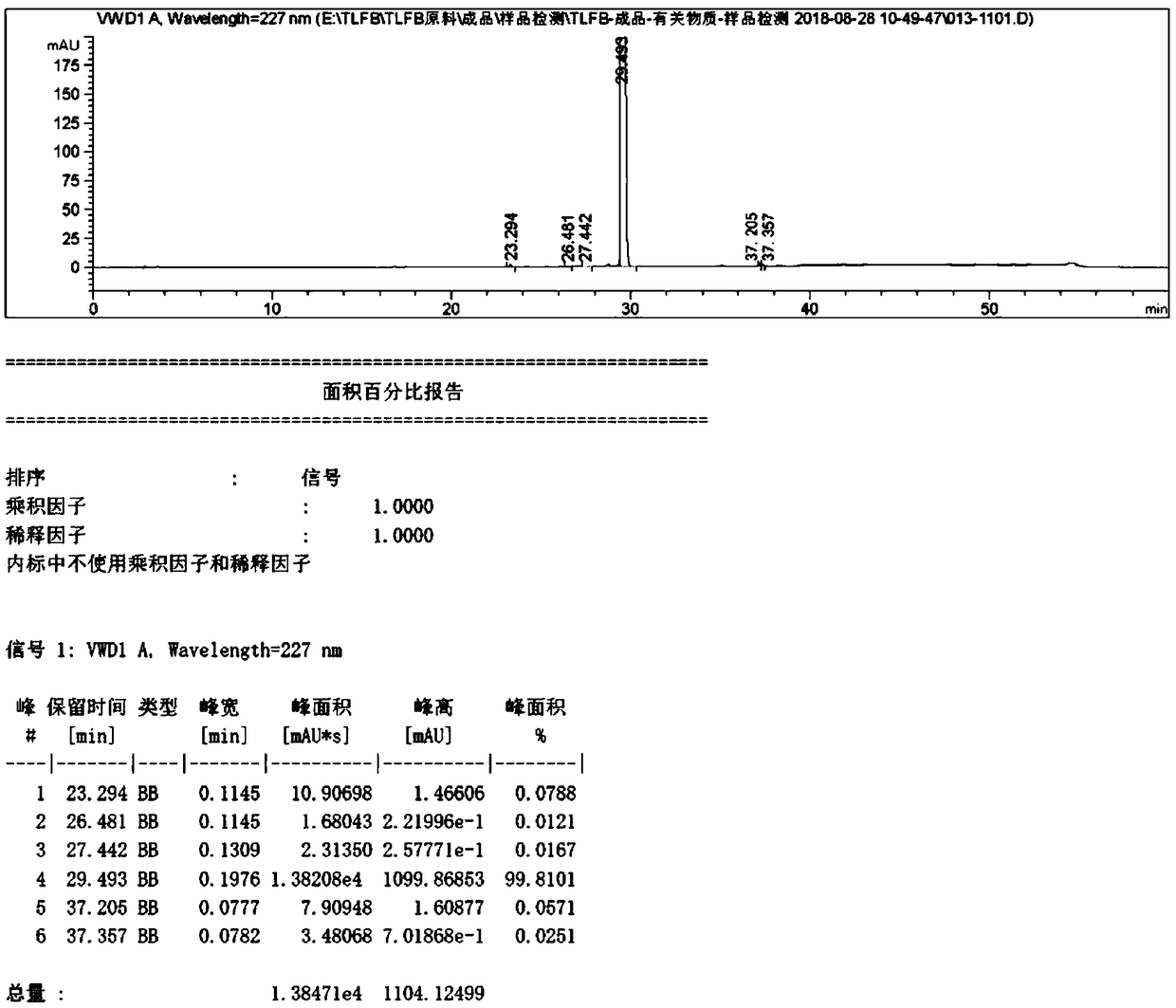
[0048]Compound (I) N-(n-butylsulfonyl)-O-[4-(4-piperidinyl)butyl]-L-tyrosine (25g, 0.057mol) described in step S1 was added to 500ml , in 0.2mol / L dilute hydrochloric acid solution, raise the temperature to 25°C, and fully stir until it dissolves. Add 1.25g of activated carbon, raise the temperature to 40°C, stir thoroughly for 30min, and filter. The filtrate kept at 40°C was extracted and washed with 250 ml of ethyl acetate for 30 min, and the ethyl acetate phase was separated. After maintaining the temperature of the prepared product solution at 25° C., drop it into 250 ml of 5 mol / L hydrochloric acid solution, stir and crystallize for 5 hours, and then filter and dry. 22.87 g of white solid was obtained, the yield was 81.3%, and the HPLC purity was 99.81%. According to the present embodiment, tirofiban hydrochloride is obtained and the high-performance liquid chromatogram of HPLC detection is as follows: image 3 shown.
[0049] For the technical solutions of the same p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com