HPLC characteristic map of Sanjin preparation and its construction method
A characteristic map and construction method technology, applied in the direction of instruments, measuring devices, scientific instruments, etc., can solve the problems that cannot truly reflect the quality of medicinal materials, and cannot evaluate the main material basis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、 3
[0043] Embodiment 1, the establishment of the high-performance liquid chromatography of Sanjin preparation HPLC characteristic collection of spectra
[0044] 1. Instruments and reagents
[0045] 1.1 Instrument
[0046] Waters2695 high-performance liquid chromatography, Waters2998 detector; METTLER TTOLEDO XP404S electronic balance; BRANSON S7500 ultrasonic cleaner.
[0047] 1.2 Reagent
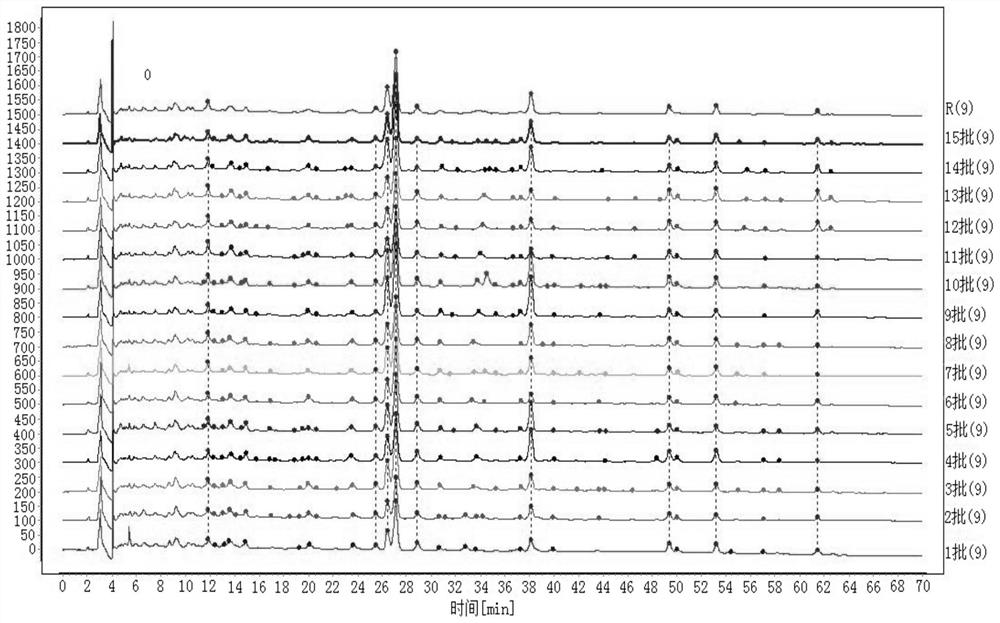
[0048] The reference substance asiaticoside (batch number: 110892-201505, purity: 91.2%) was provided by China National Institutes for Food and Drug Control. 15 batches of Sanjin Tablets were produced by Guilin Sanjin Pharmaceutical Co., Ltd., with batch numbers 1602001, 1604011, 1606001, 1608001, 1610001, 1503002, 1505001, 1507002, 1509003, 1512002, 170227, 170308, 170304, 43007. Acetonitrile was imported chromatographically pure, water was ultrapure water, and the rest of the reagents were analytically pure.
[0049] 2. Methods and results
[0050] 2.1 Chromatographic conditions
[005...
Embodiment 2
[0073] Example 2. Methodological investigation of high performance liquid chromatography for establishing the HPLC characteristic spectrum of Sanjin preparation
[0074] The high performance liquid chromatography of the HPLC characteristic spectrum of the Sanjin preparation established in Example 1 was investigated in the following aspects.
[0075] 1. Precision test
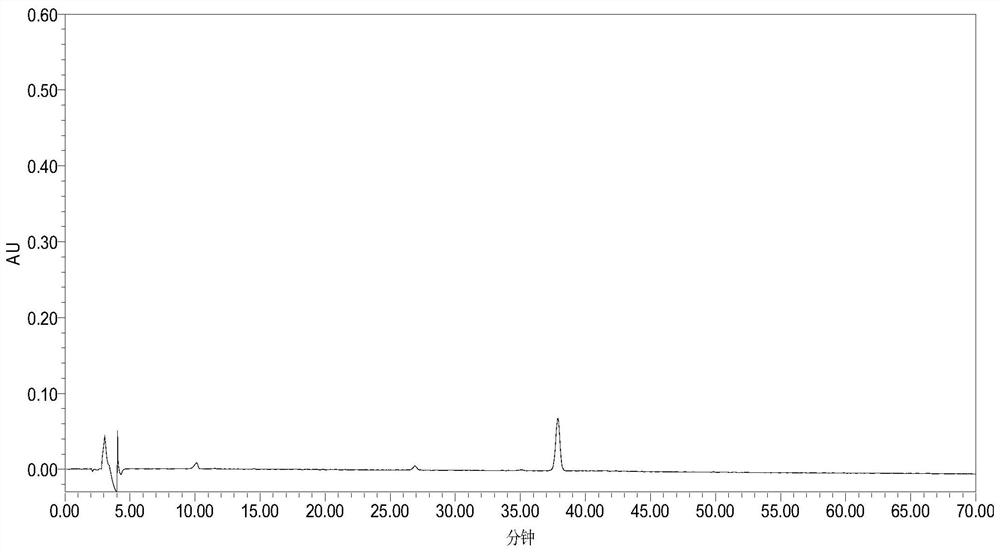
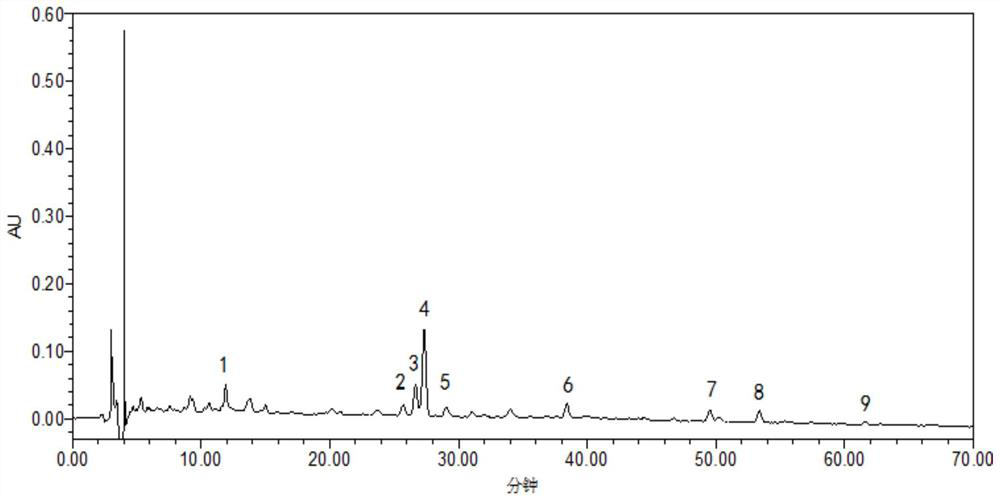
[0076] Get same batch of samples (batch number 170227), prepare need testing solution by the preparation method of need testing solution in embodiment 1, test under chromatographic conditions in embodiment 1, continuous sampling 6 times, record chromatogram, visible 6 Peak No. 1 is the reference substance Asiaticoside, the peak time is in the middle, the surrounding interference is small, and the content is relatively high, so the peak of Asiaticoside is selected as the reference peak S, and the "Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System (V2 .0)" for evaluation. The results show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com