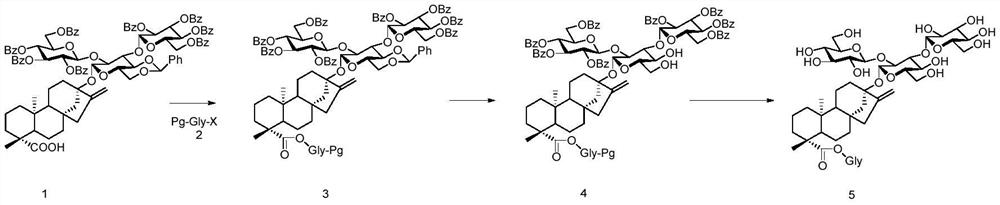
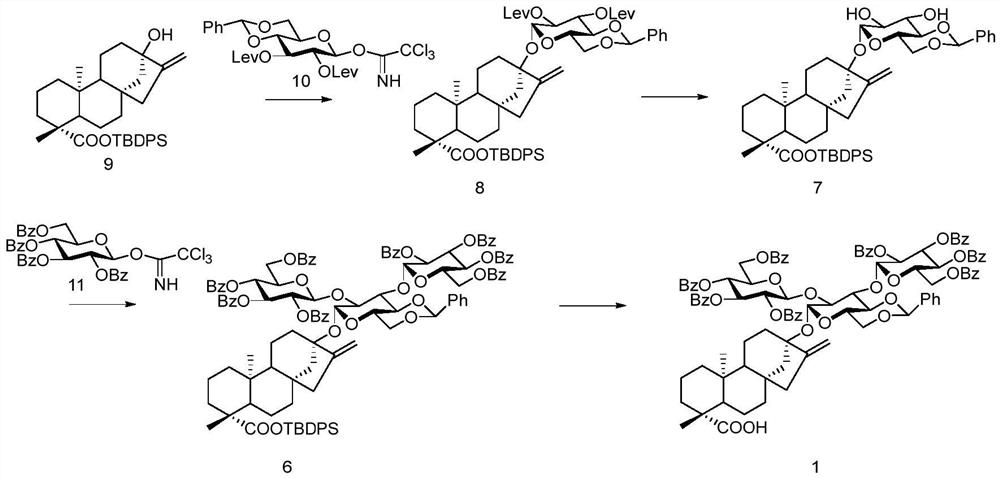
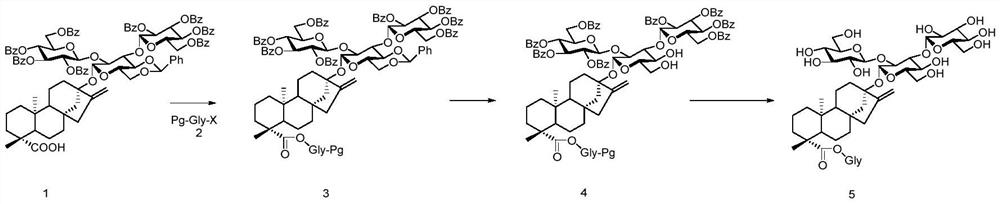
A kind of preparation method of stevioside
A technology of steviol glycoside and glycosidation reaction, which is applied in the field of stevioside preparation and can solve problems such as unreasonableness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of some compounds in the present invention is as follows:
[0052] 1. The preparation method of compound 9:
[0053]
[0054] We started from commercially available steviol, reacted with TBDPSCl under alkaline conditions, and obtained the receptor compound 9 required for our reaction through the conventional reaction conditions in this field.
[0055] 9: [α] D25 = -31.6 (c 1, CHCl3); 1H NMR (400MHz, CDCl3) δ7.69-7.66 (m, 4H), 7.45-7.40 (m, 2H), 7.38-7.34 (m, 4H ),2.93(d,J=4.8Hz,1H),2.78(d,J=4.8Hz,1H), 2.34(brs,1H),2.24-2.19(m,1H),2.14(dd,J=2.0, 11.2Hz, 1H), 1.35(dd, J=2.8, 11.2Hz, 1H), 1.27(s, 3H), 1.14(s, 9H), 0.76(s, 3H); 13C NMR(100MHz, CDCl3) δ176. 9,135.8 (2C),132.1(2C),130.1,127.7,74.9,65.4,57.1,53.9,48.8,46.7,45.8,45.2,41.7,41.5,40.8,39.5,38.6,34.8,29.3,27.3,22.3,19.7, 19.4, 19.3, 16.4, HRMS (ESI) calcd for C18H21IO8Na[M+Na]+595.3214, found 595.3210.
[0056] 2. The preparation method of compound 2-1:
[0057] Under nitrogen protect...
Embodiment 1
[0071] Embodiment 1: the preparation of Reb A
[0072]
[0073] Step (1): Compound 1 (110 mg, 0.064 mmol) was dissolved in CHCl 3 / H 2 O (4mL, v / v=1:1), add K at room temperature 2 CO 3 (26.4mg, 0.2mmol) and TBAB (41mg, 0.13mmol), stirred at room temperature for 10min, then added compound 2-1 (0.84mg, 0.13mmol) into the reaction system, reacted at 40°C for 14h, TLC detected that the reaction was complete , diluted with EA, washed three times with water, washed once with saturated sodium chloride, dried the organic phase with anhydrous sodium sulfate, filtered and suspended to dryness, column chromatography (PE / EA=1.5:1), the white foam compound 3-1 (125mg), Yield: 85%.
[0074] 3-1: [α] D 25 =+34.3(c 1,CHCl 3 ); 1 H NMR (400MHz, acetone-d 6 )δ8.16-8.11(m, 4H), 8.04-7.83(m, 17H), 7.64-7.28(m, 44H), 6.44(d, J=8.0Hz, 1H), 6.11(t, J=9.6Hz ,1H),6.05-5.99(m,2H),5.90(t,J=9.2Hz,1H),5.81(dd,J=8.0,10.0Hz,1H),5.76-5.66(m,4H),5.63( dd,J=7.6,9.6Hz,1H),5.52(d,J=8.0Hz,1H),5.40(...
Embodiment 2
[0091] Embodiment 2: the preparation of Reb D
[0092] The preparation of Reb D refers to the preparation of Reb A, the difference is that the sugar used is diglucoside 2-2, the reaction yield of step (1) is: 93%, the total of the two steps of step (2) and (3) Yield: 81%.
[0093] Reb D: [α] D 25 =-22.0(c 0.5, MeOH); 1 H NMR (400MHz, pyridine-d 5 )δ6.37(d, J=6.4Hz, 1H), 5.83(s, 2H), 5.69(s, 1H), 5.63(d, J=7.6Hz, 1H), 5.53(d, J=7.6Hz, 1H), 5.45(d, J=7.6Hz, 1H), 5.14(d, J=7.2Hz, 1H), 5.02(s, 1H), 4.64-3.89(m, 23H), 2.77(d, J=12.8 Hz,1H),2.54(d,J=11.2Hz,1H),2.28-1.68(m,13H),1.43(s,3H),1.16(s,3H), 1.01(d,J=12.0Hz,1H ),0.89(d,J=6.4Hz,1H),0.77(dd,J=10.4,14.4Hz,1H); 13 C NMR (100MHz, pyridine-d 5 )δ175.8, 154.0, 105.7, 104.8, 104.6, 104.5, 97.8, 93.6, 88.1, 86.6, 81.0, 80.8, 79.0, 78.6, 78.4 (2C), 78.2, 78.1, 78.0, 77.4, 76.4, 76.2, 75.3, 72. 72.0, 71.6, 70.8, 69.9, 63.2, 63.0, 62.3(2C), 62.1, 57.4, 53.9, 49.7, 47.6, 44.3, 44.1, 42.2, 41.8, 40.6, 39.7, 37.8, 29.2, 22.2, 20.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com