Method for activating peroxysulphate and degrading pollutant, and application
A technology for activating persulfate and persulfate, which is applied in the field of pollutant treatment, can solve the problems that COD and TN cannot be removed simultaneously, and the removal of nitrate nitrogen cannot be realized, and has good application prospects, excellent degradation effect, and improved degradation efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1) Selection of iron ion source:
[0046] The source of ferrous iron selected in this embodiment is ferrous sulfate heptahydrate.
[0047] 2) Selection of persulfate:
[0048] The persulfate selected in this embodiment is sodium persulfate.
[0049] 3) Selection of complexing agent: ascorbic acid
[0050] 4) The specific degradation process is implemented according to the following steps:
[0051] Get the p-chlorophenol aqueous solution (concentration is 20mg / L) in the scope of adjusting pH value 10-12, add ascorbic acid: Fe 2+ : The molar ratio of sodium persulfate is 1:10:8, the total volume is 50ml, and the reaction degradation is carried out at 30°C and 200rpm shaking table for 25min;
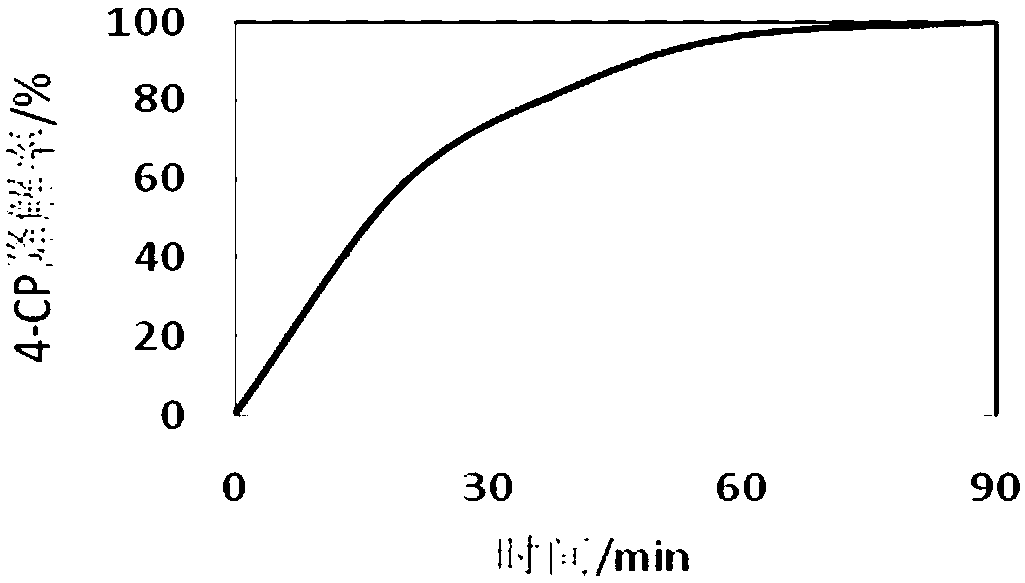
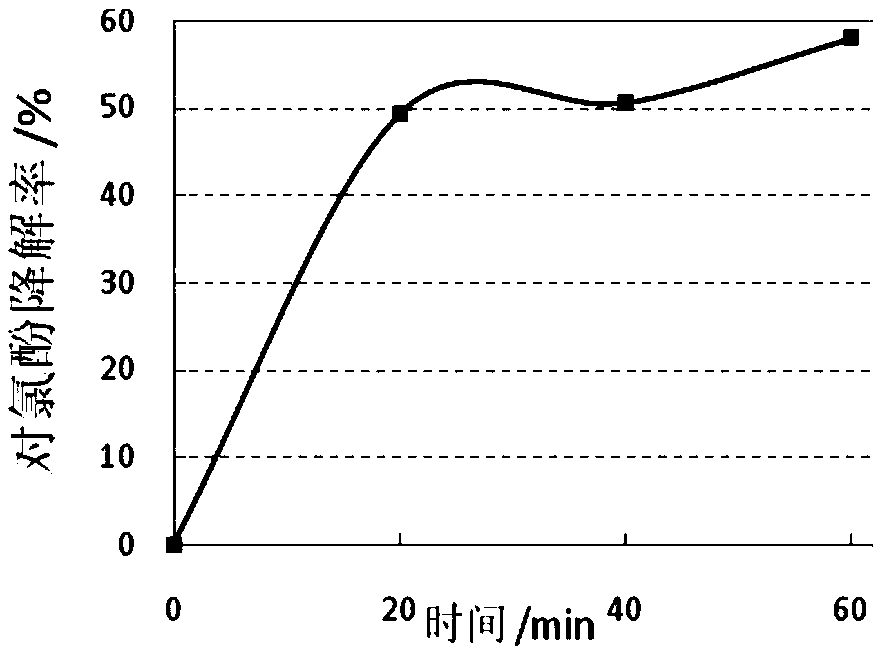
[0052] Take 3ml of the degraded solution in a 10ml centrifuge tube, extract with an equal volume of ethyl acetate, pass through a 0.22um water filter membrane, and detect the content of p-chlorophenol (4-CP) with high performance liquid phase. It was found that after 20min of 4- ...
Embodiment 2
[0054] 1) Selection of iron ion source:
[0055] The source of ferrous iron selected in this embodiment is ferrous acetate and ferrous sulfate heptahydrate.
[0056] 2) Selection of persulfate:
[0057] The persulfate selected in this embodiment is sodium persulfate.
[0058] 3) Selection of complexing agent: ascorbic acid
[0059] 4) The specific degradation process is implemented according to the following steps:
[0060] Get the p-chlorophenol aqueous solution (concentration is 20mg / L) that adjusts pH value in the scope of 8-9, add ascorbic acid: Fe 2+ : The molar ratio of sodium persulfate is 5:1:1, the total volume is 50ml, and the reaction degradation is carried out at 35°C and 210rpm shaking table for 30min;
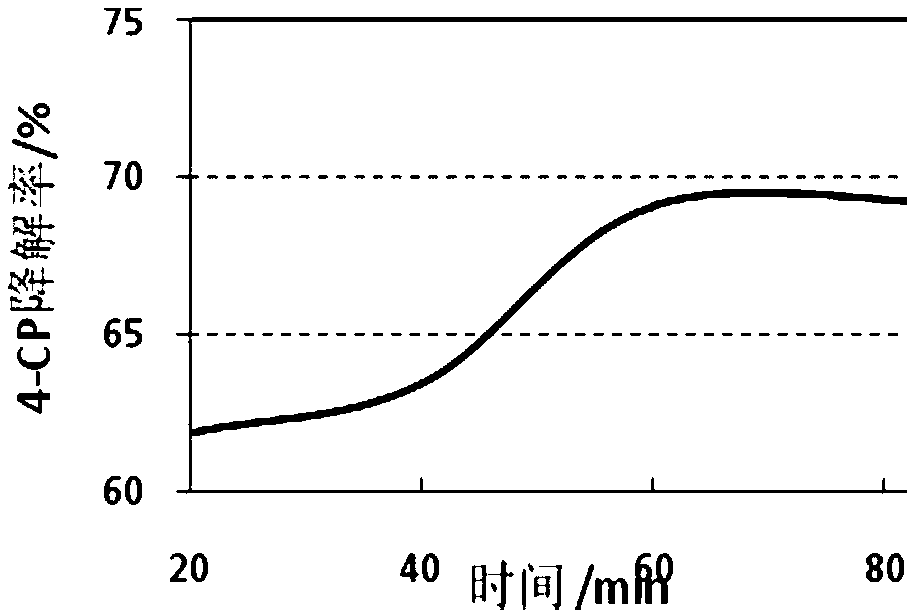
[0061] Get the solution after the reaction degradation of 3ml in the 10ml centrifuge tube, extract with equal volume of ethyl acetate, pass through the 0.22um water system filter membrane, detect the content of p-chlorophenol (4-CP) with high performance liqui...
Embodiment 3
[0063] 1) Selection of iron ion source:
[0064] The source of ferrous iron selected in this embodiment is ferrous chloride tetrahydrate.
[0065] 2) Selection of persulfate:
[0066] The persulfates selected in this embodiment are potassium persulfate and sodium persulfate.
[0067] 3) Selection of complexing agent: ascorbic acid
[0068] 4) The specific degradation process is implemented according to the following steps:
[0069] Get the p-chlorophenol aqueous solution (concentration is 20mg / L) in the scope of adjusting pH value 2-4, add ascorbic acid: Fe 2+ : The molar ratio of sodium persulfate is 2:4:7, the total volume is 50ml, and the reaction degradation is carried out at 20°C and shaken at 250rpm for 60min;
[0070] Take 3ml of the degraded solution in a 10ml centrifuge tube, extract with an equal volume of ethyl acetate, pass through a 0.22um water filter membrane, and use HPLC to detect that the degradation rate of p-chlorophenol (4-CP) is 98% for 20 minutes. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com