Application of click chemistry and declick chemistry to quantitative synthesis and release of drug
A technology of click chemistry and medicine, applied in chemical instruments and methods, organic chemistry, compounds containing elements of group 3/13 of the periodic table, etc., to achieve mild reaction conditions, high reaction yield, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
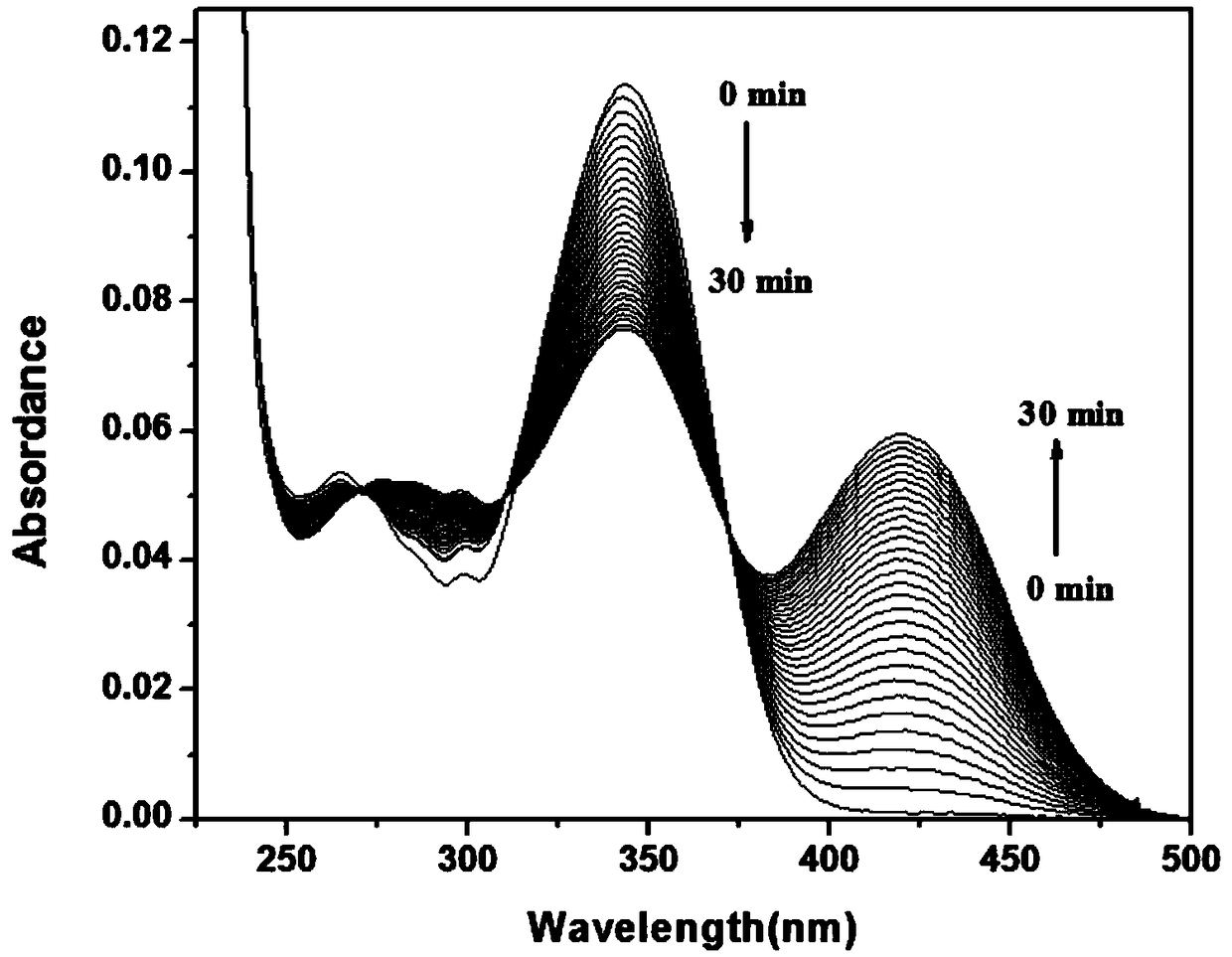
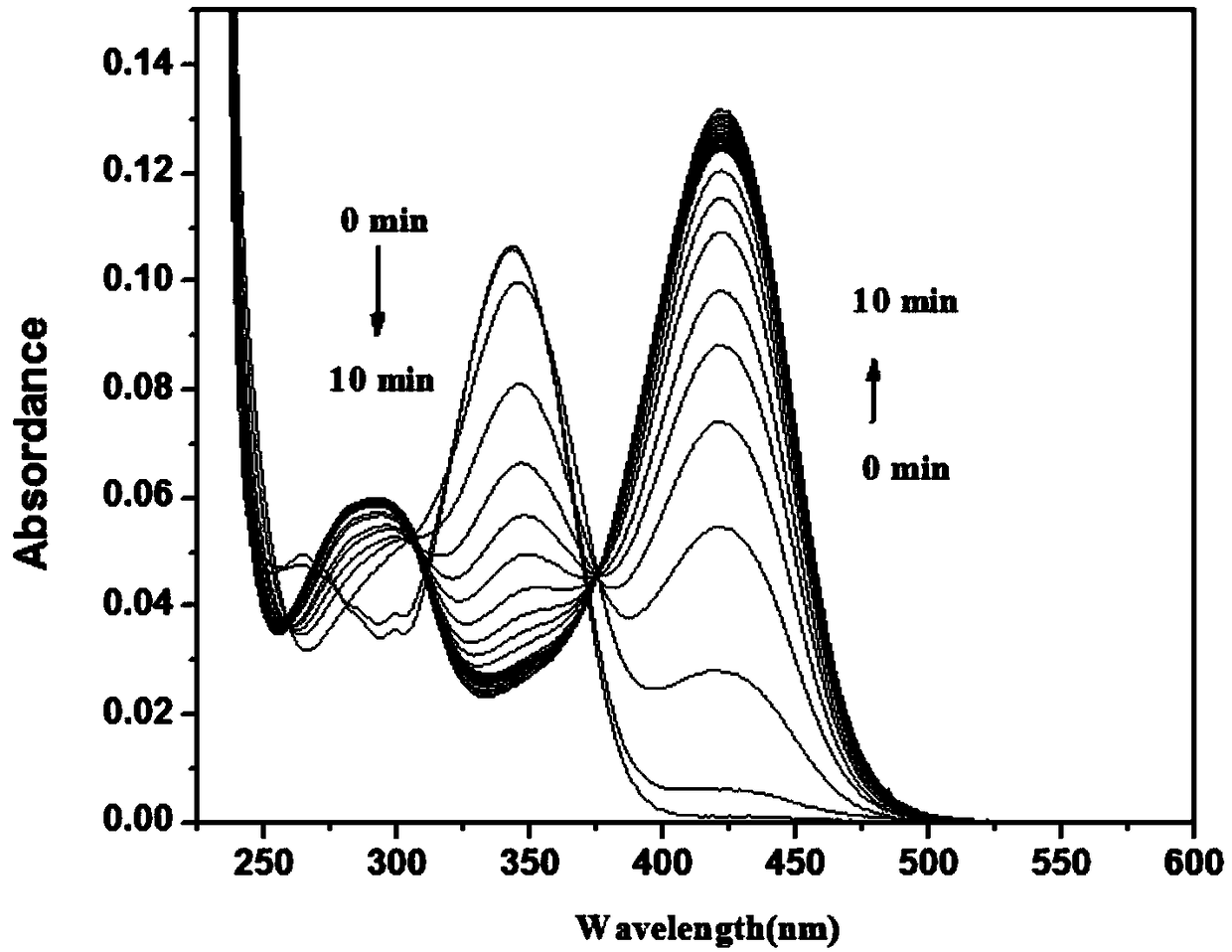
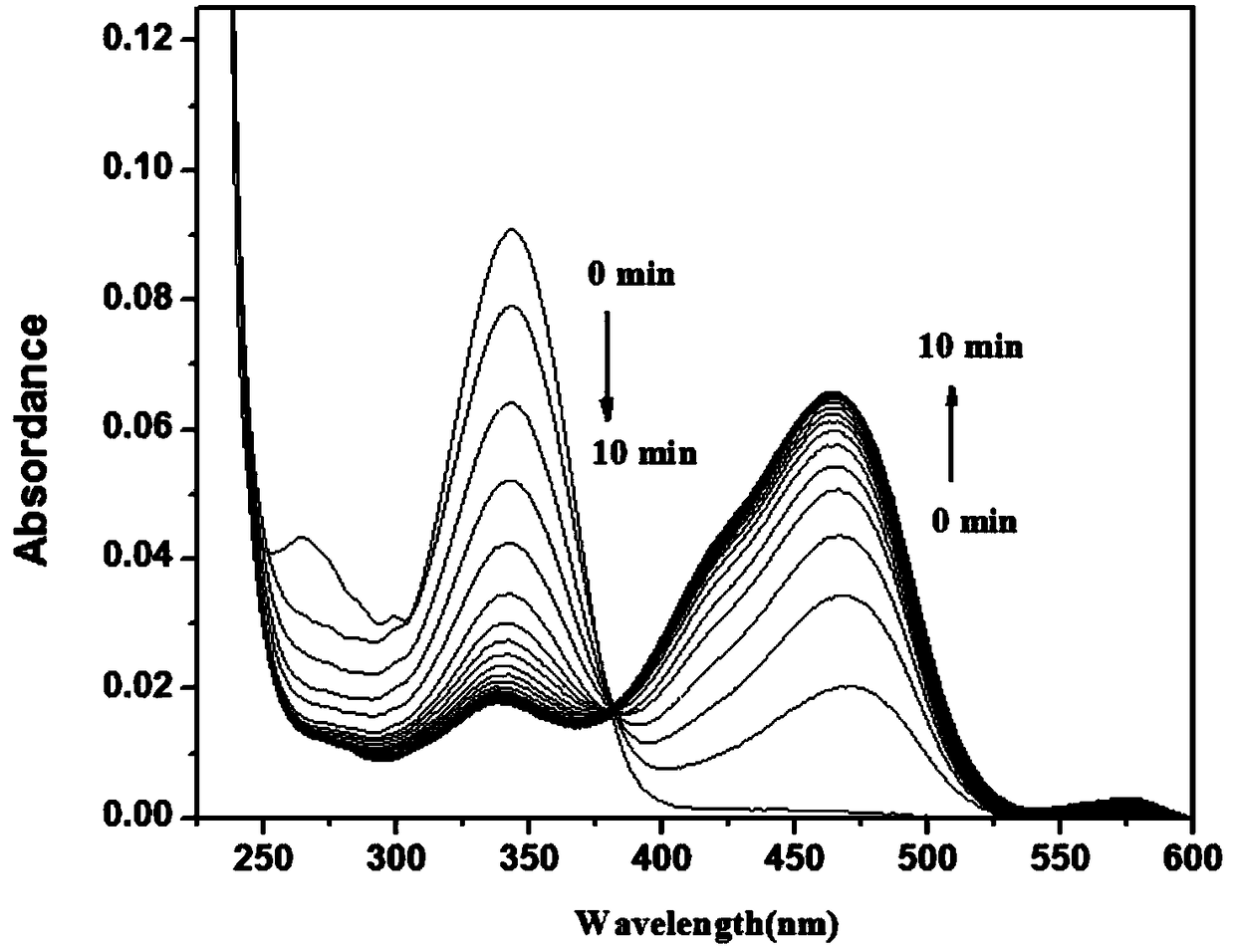
[0071] To 2 mL of PB buffer (10 mM, pH=7.4, 1 mM CTAB), add 10 μL of NBD-Cl stock solution with a concentration of 2 mM, and then add 1 μL of GSH with a concentration of 20 mM, and test the change of its absorption spectrum with time, as shown in figure 2 As shown, the absorption at 343nm decreased gradually, the absorption at 421nm increased gradually, and the reaction equilibrium was reached in about 3 minutes. After the reaction reaches equilibrium, add 10 μL of Cys or Hcy with a concentration of 20 mM, such as Figure 6 , as shown in 7, the absorption at 421nm decreases gradually, and the absorption peak at 470nm gradually increases, and reaches equilibrium in about 3 minutes, as Figure 15 ,16, the absorption spectrum when it reaches equilibrium is basically the same as the absorption spectrum when NBD-Cl (10 μM) is added to Cys or Hcy (10 μM).
[0072] To 2 mL of PB buffer (10 mM, pH=7.4, 1 mM CTAB) system, add 10 μL of NBD-Cl stock solution with a concentration of 2 m...
Embodiment 2
[0074] To 2mL of PB buffer (10mM, pH=7.4, 2.5mM CTAB) system, add 10μL of NBD-Cl stock solution with a concentration of 2mM, and then add 1μL of GSH with a concentration of 20mM, and test the change of its absorption spectrum with time, 343nm The absorption at 421nm decreased gradually, and the absorption at 421nm increased gradually, and the reaction equilibrium was reached in about 3 minutes. After the reaction reaches equilibrium, add 10 μL of Cys or Hcy with a concentration of 20mM, the absorption at 421nm gradually decreases, the absorption peak at 470nm gradually increases, and reaches equilibrium in about 3 minutes. The absorption spectrum when it reaches equilibrium is the same as that of NBD-Cl( 10μM) when Cys or Hcy (10μM) was added, the absorption spectra were basically the same.
[0075] To 2mL of PB buffer (10mM, pH=7.4, 1mM CTAB) system, add 10μL of NBD-Cl stock solution with a concentration of 2mM, then add 1μL of GSH with a concentration of 20mM, and test the c...
Embodiment 3
[0077] To 2mL of PB buffer (10mM, pH=7.4, 5mM CTAB) system, add 10μL of NBD-Cl stock solution with a concentration of 2mM, then add 1μL of GSH with a concentration of 20mM, and test the change of its absorption spectrum with time, at 343nm The absorption at 421nm gradually decreased, and the absorption at 421nm gradually increased, and the reaction equilibrium was reached in about 3 minutes. After the reaction reaches equilibrium, add 10 μL of Cys or Hcy with a concentration of 20mM, the absorption at 421nm gradually decreases, the absorption peak at 470nm gradually increases, and reaches equilibrium in about 3 minutes. The absorption spectrum when it reaches equilibrium is the same as that of NBD-Cl( 10μM) when Cys or Hcy (10μM) was added, the absorption spectra were basically the same.
[0078]To 2mL of PB buffer (10mM, pH=7.4, 1mM CTAB) system, add 10μL of NBD-Cl stock solution with a concentration of 2mM, then add 1μL of GSH with a concentration of 20mM, and test the chang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com