A kind of anti-human csf-1r monoclonal antibody and its application
A technology of CSF-1R and monoclonal antibody, applied in the fields of antibody, application, anti-animal/human immunoglobulin, etc., can solve problems that need further research and development, and achieve the effect of novel sequence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 obtains the mouse monoclonal antibody of specific anti-CSF1R by fusion hybridoma technology
[0036] 1.1 Animal immunity
[0037] Mice were immunized according to the general method in the literature (E Harlow, D. Lane, Antibody: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1998). The immunogen is a recombinant human CSF1R (amino acid interval I l e20-Glu 512) protein (Acrobiosystems, Cat#CSR-H5258) containing a human IgG1Fc tag at the C-terminus. Recombinant human CSF1R protein with his s tag (Acro biosystems, cat#CSR-H5228) was used as the detection antigen for determination of serum titer and hybridoma screening. Briefly, remove an appropriate amount of Freund's adjuvant into a 1.5ml EP tube, and shake to mix. Prepare the antigenic protein solution with PBS. Mix the adjuvant and protein antigen solution according to the required amount, fully emulsify the antigen by pushing each other through the syringe to form ...
Embodiment 2
[0043] Example 2 In vitro assay method for measuring CSF1R monoclonal antibody functional activity
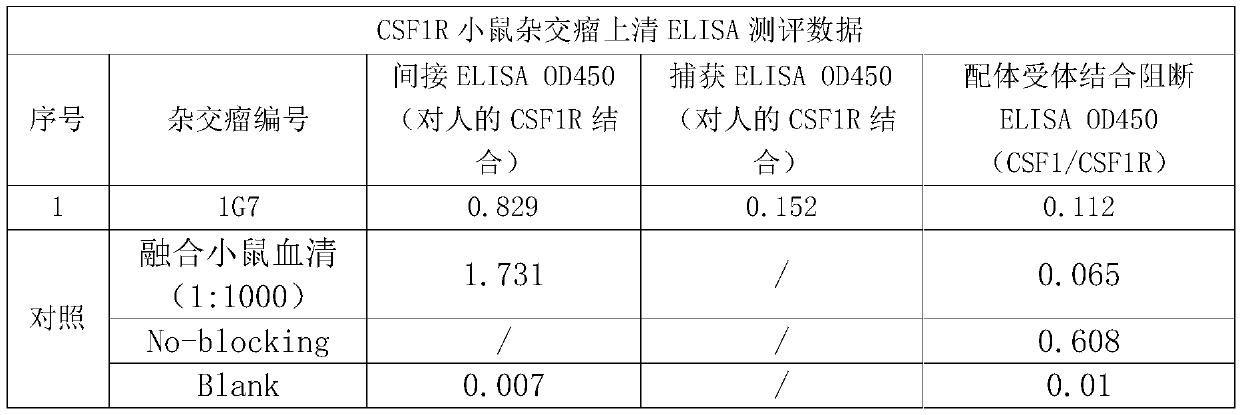
[0044] 2.1 Determination of antibody binding ability based on capture ELSIA Prepare goat anti-mouse IgG Fcr-specific secondary antibody (Jackson Immuno Research, #115-006-071) with 1xPBS, so that the final concentration is 2 μg / ml, add liquid at 100 μl / well In 96-well ELISA plate, coated overnight at 4 degrees. The next day, after washing the plate 4 times with PBS solution containing 0.05% Tween 20 (ie 1xPBST), 200 μl / well of 5% skim milk powder in PBST solution was added and placed at 37°C for 2 hours to block. Wash the plate again, add 100 μl / well of diluted antibody solution or hybridoma supernatant, incubate at 37 degrees for 40 minutes, and then wash the plate 4 times. Add the prepared 60 nM biotin-labeled human CSF1R Fc protein solution (in 2.5% skimmed milk powder in PBST) at 100 μl / well, incubate at 37°C for 40 minutes, and then wash the plate 4 times. Add 1:10000 di...
Embodiment 3
[0053] Example 3 Binding activity of anti-CSF1R mouse monoclonal antibody
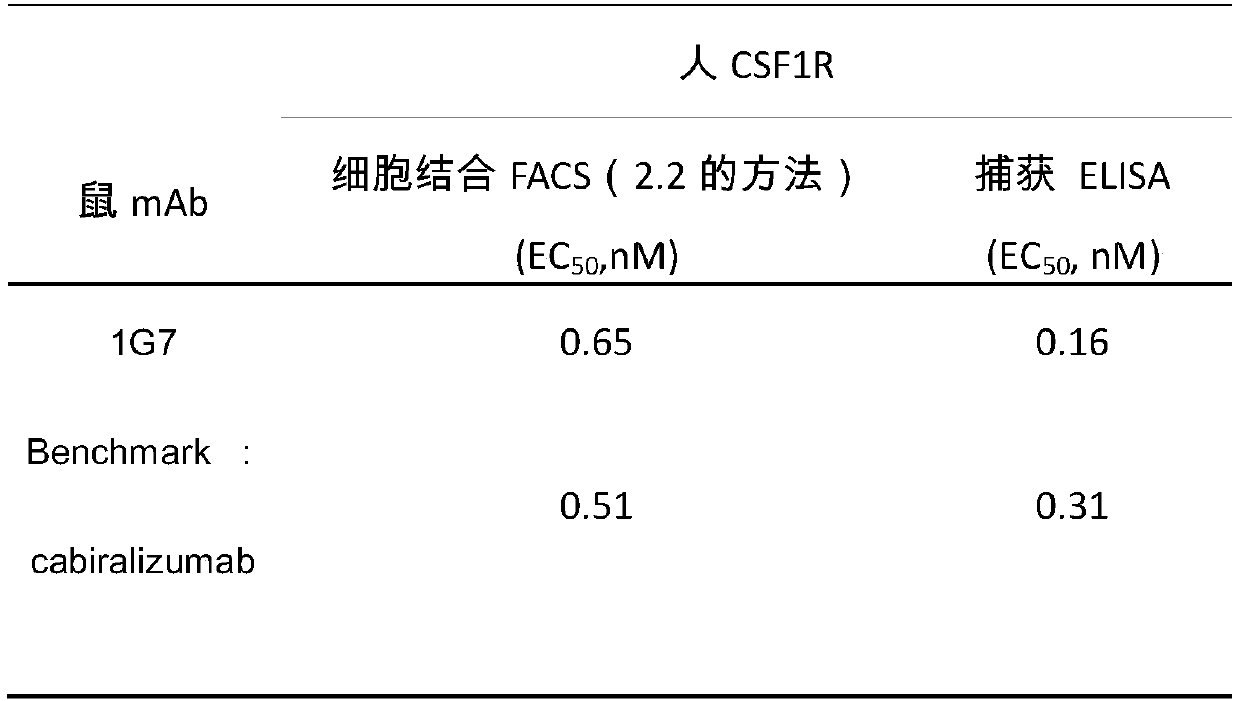
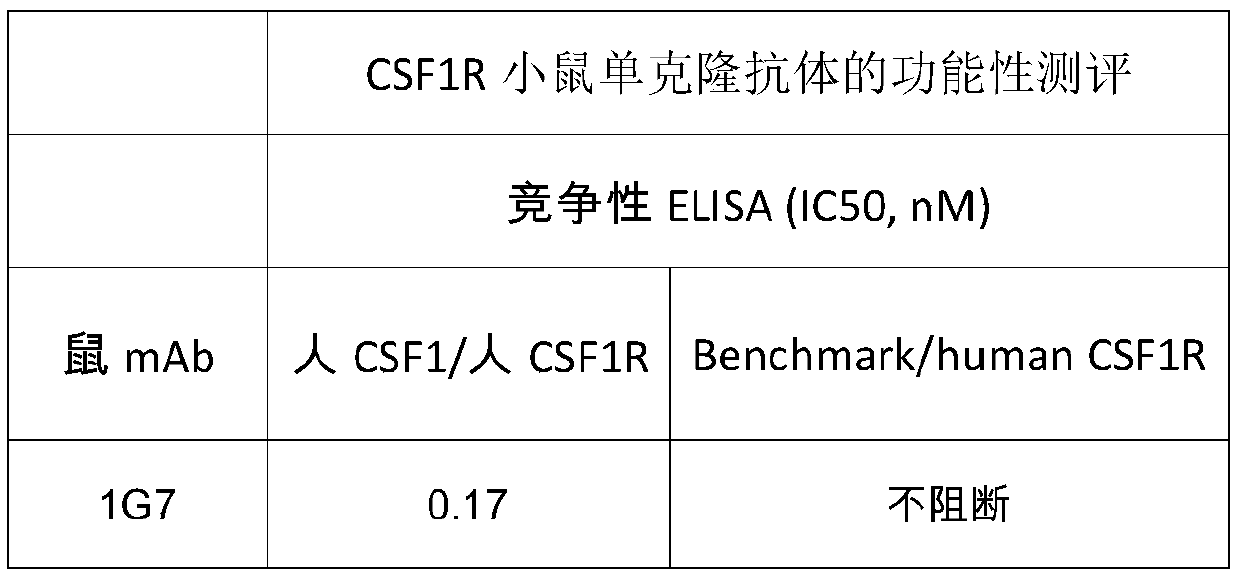
[0054] According to the analysis method described in Example 2, the evaluation of the binding activity of the CSF1R antibody is summarized in Table 2 below. Among them, Benchmark (Cabiralizumab) is used as a control, which is an existing non-commercialized anti-human CSF1R monoclonal antibody. It can be seen from Table 2 that the binding activity of the anti-human CSF-1R monoclonal antibody of the present invention to human CSF1R antigen is significantly better than that of Benchmark.
[0055] Table 2 Binding activity of CSF1R antibody
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com