Allergen-specific IgE antibody quality control products and preparation method thereof
A quality control product, allergen technology, applied in material inspection products, measuring devices, instruments, etc., can solve the problems of inconsistent test results, batch differences, complex components, etc. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
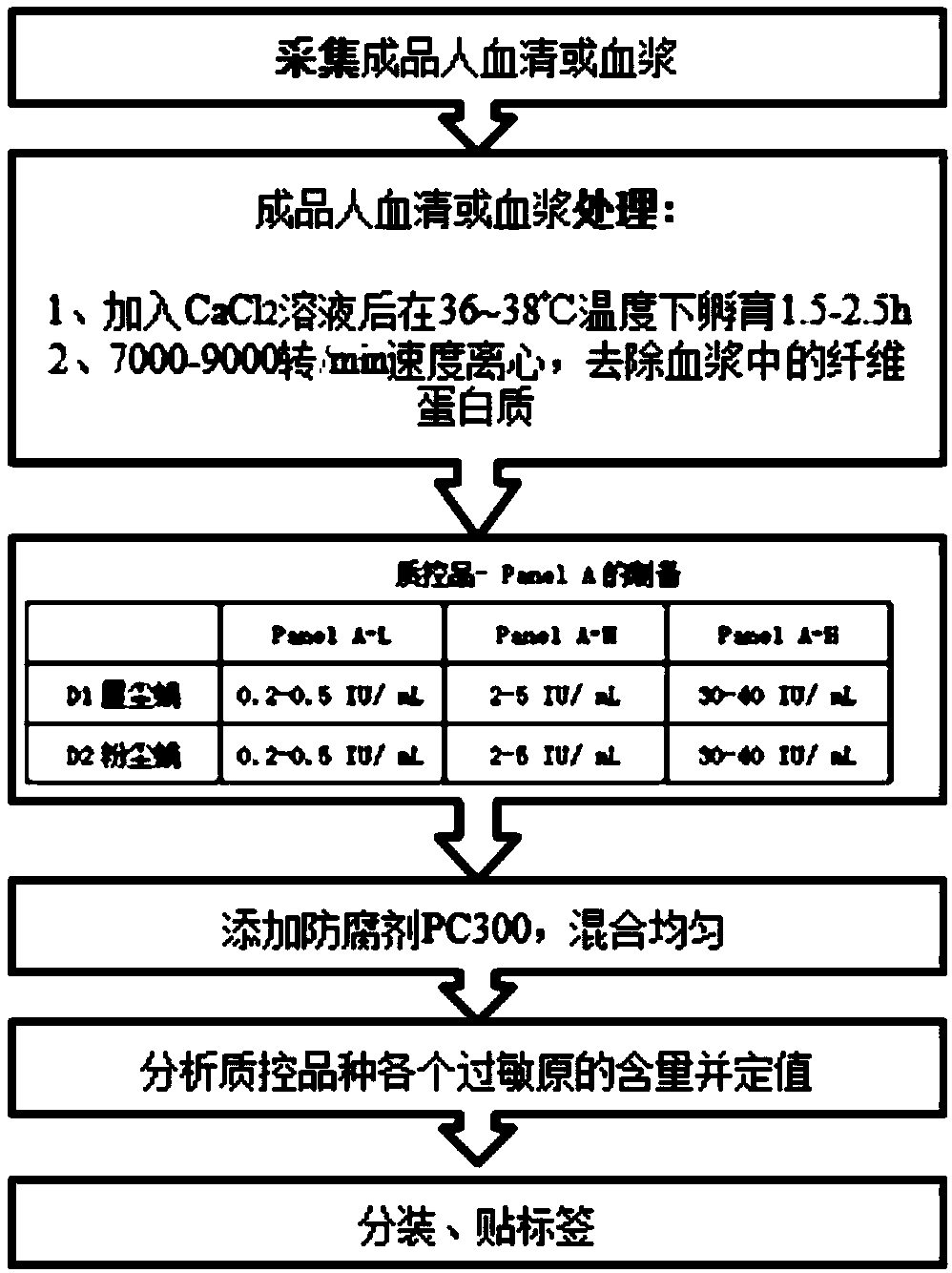
[0032] Embodiment 1, preparation flow see figure 1 .
[0033] (1) Collect finished human serum or plasma, select the finished human plasma with sufficient sources and easy to obtain as the material, the composition of the organic matter after plasma treatment is completely consistent with the serum in theory, and the amount of additives and preparations is small, and the difference between bottles is small;
[0034] (2) Adding CaCl to finished human serum or plasma 2 After the solution was incubated at 36-38°C for 2 hours, centrifuged at a speed of 7000-9000 rpm to remove fibrin in the plasma; (3) Prepare corresponding quality control plates according to the requirements of quality control products;
[0035] (4) Add preservative (PC300), the addition ratio is 0.05% of the volume of finished human serum or plasma, and mix well;
[0036] (5) Analyze the content of each allergen in the quality control product and determine the value;
[0037] (6) Packing and labeling.
[0038...
Embodiment 2
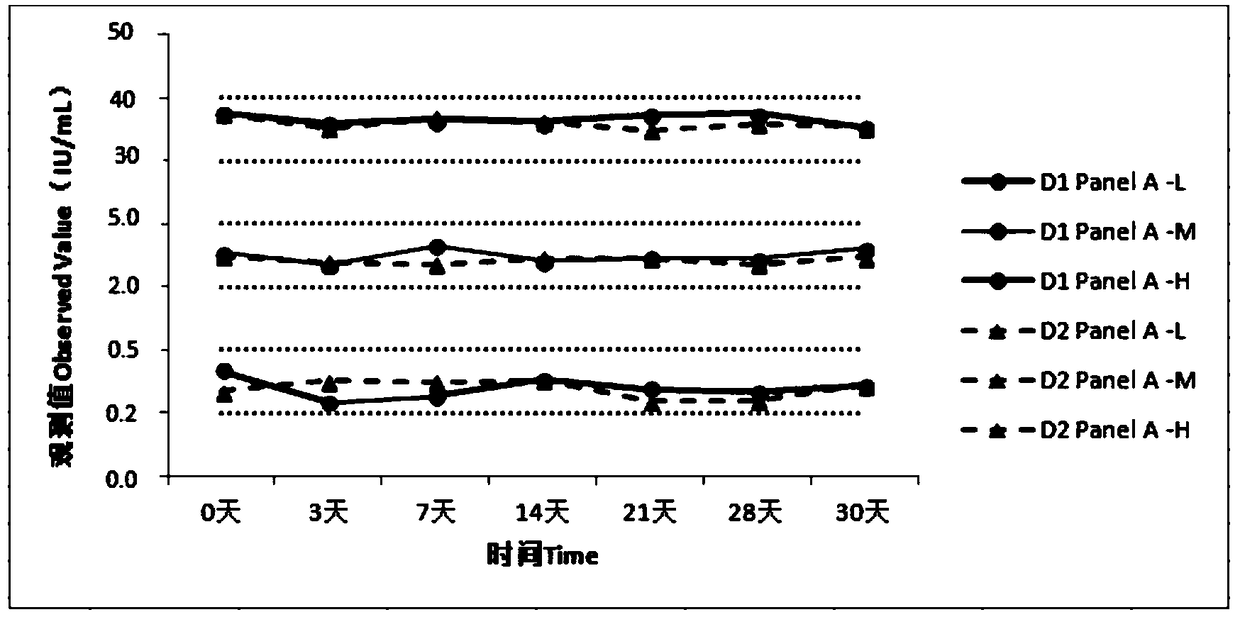
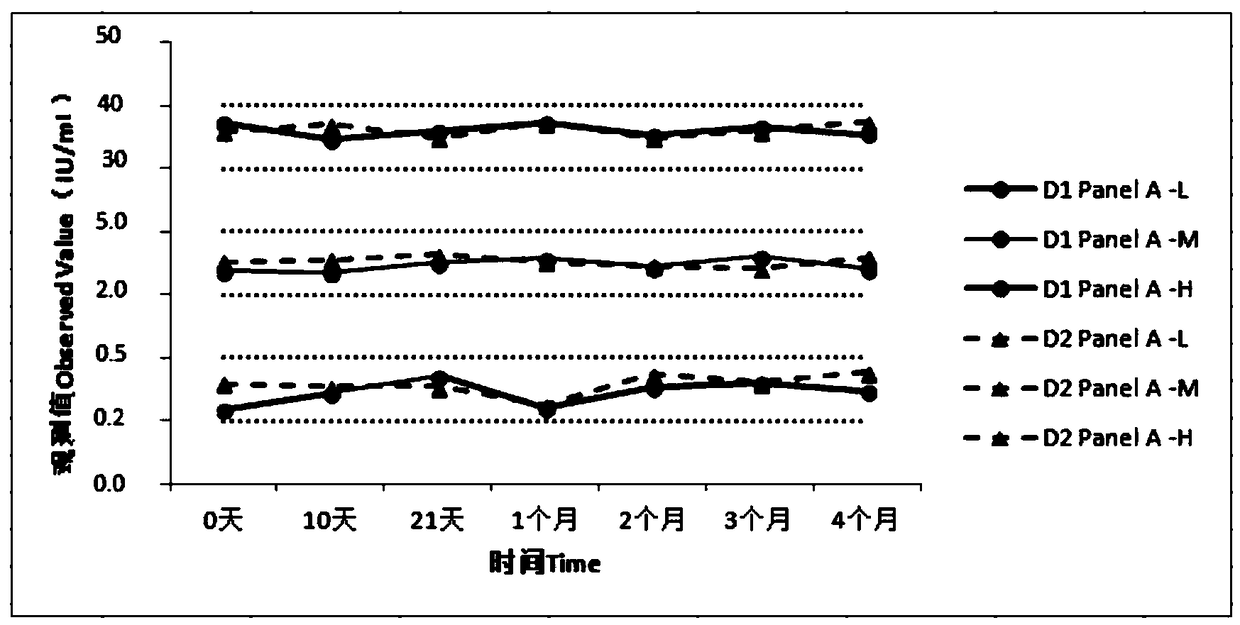
[0039] Embodiment 2. Perform performance test on the quality control product-Panel A prepared in embodiment 1.
[0040] 1. In-bottle repeatability
[0041] (1) Test method: For each bottle of quality control product-Panel A (Panel A-L, Panel A-M, Panel A-H), measure continuously 10 times respectively, and calculate the average value of the measurement results and standard deviation S1, calculate the coefficient of variation in the bottle according to the following formula
[0042] (2) Test requirements: CV≤10%.
[0043] (3) Test results:
[0044]
[0045] (4) Result description: the repeatability in the quality control panel Panel-A bottle is ≤10%;
[0046] 2. Difference between bottles
[0047] (1) Test method: Take the repeated quality control products in the bottle - Panel A (Panel A-L, Panel A-M, Panel A-H) and other quality control products of the same batch number - Panel A (Panel A-L, Panel A-M, Panel A-H) four bottle, for the five bottles of quality control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com