Pharmaceutical composition containing angiotensin receptor blocker
A technology of receptor antagonists and angiotensin, which is applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, and pharmaceutical formulations to achieve the effect of increasing bioavailability and improving dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0032] Experimental example 1. Solubility improvement effect of valsartan
[0033] Compositions of Examples 1 to 4 and Comparative Examples 1 to 2 of Table 1 below were prepared.
[0034] In the case of using only a dispersant, the main component is dispersed in the dispersant.
[0035] Add the main ingredient to the mixture of dispersant and dissolution aid, and mix until the main ingredient is completely dispersed.
[0036] When the polymer is also mixed, the polymer is added while the mixture of the dispersant and the dissolution aid is heated, mixed completely and cooled, then the main component is added and mixed until the main component is completely dispersed.
[0037] Unless otherwise stated, other comparative examples and examples described below were produced by the same or similar production steps as above.
[0038] [Table 1] Composition of Examples and Comparative Examples
[0039] (unit: mg)
[0040]
[0041] Into 50 ml of the solution and 50 ml of water ha...
experiment example 2
[0051] Experimental example 2. Solubility improvement effect of telmisartan
[0052] Compositions of Example 5 to Example 8 and Comparative Example 3 to Comparative Example 4 of Table 3 below were prepared.
[0053] At this time, the basifying agent is first mixed with the dispersant and the dissolution aid, and the polymer is charged and mixed in a heated state. After cooling the mixture in which the polymers have been completely mixed, add the main component and mix until the main component is completely dispersed.
[0054] [table 3] the composition of embodiment and comparative example
[0055] (unit: mg)
[0056]
[0057] Examples 5 to 8 and Comparative Examples 3 to 4 prepared above were put into 50 ml of solutions and 50 ml of water having pHs of 1.2, 4.0, and 6.8, respectively, in a manner corresponding to 80 mg of telmisartan. The composition was shaken for 24 hours at 25° C. and 200 rpm (VS-8480, Vision Technology Company). Each sample was centrifuged at 13,000...
experiment example 3
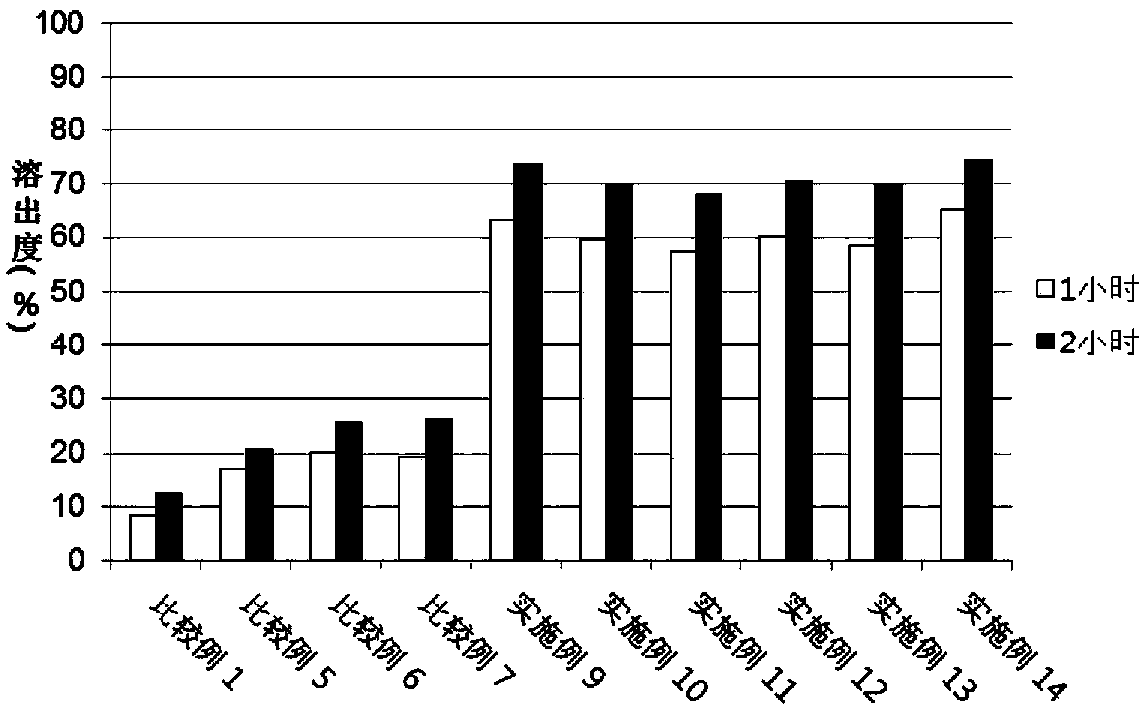
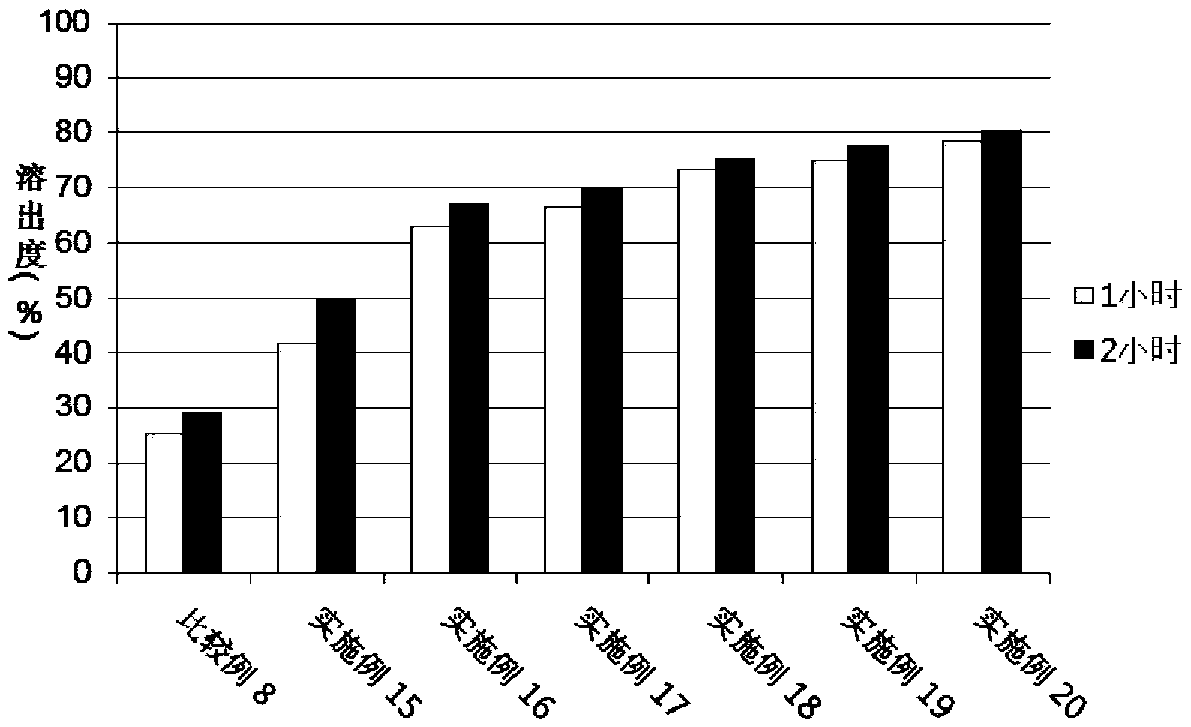
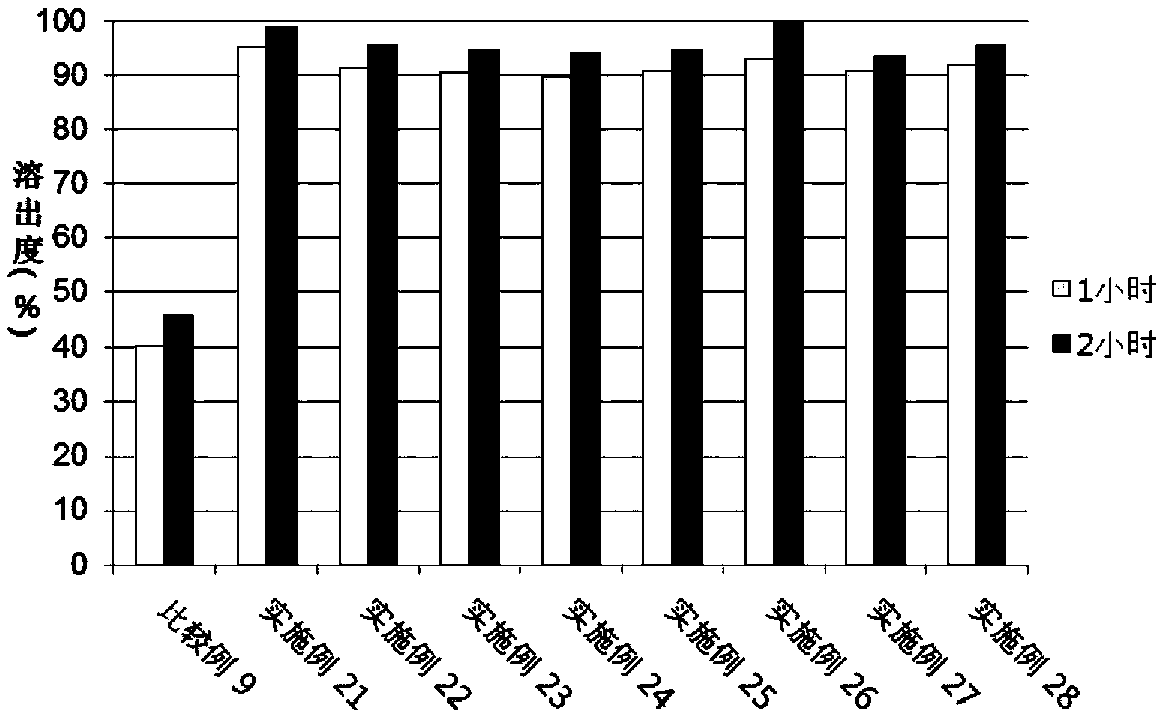
[0067] Experimental example 3. Dispersant screening
[0068] In a similar manner to Experimental Example 1 above, Examples 9 to 14 and Comparative Example 1, Comparative Example 5 to Comparative Example 7 were prepared with the composition of Table 5 below.
[0069] [Table 5] Composition of Examples and Comparative Examples
[0070] (unit: mg)
[0071]
[0072] The test was carried out according to USP40 dissolution apparatus 2 (slurry method) by using a pH 1.2 dissolution solution (Korean Pharmacopoeia Dissolution Test First Solution) in which the dissolution rate of valsartan was shown to be low, and according to the experimental The analysis conditions of Example 1 were used to analyze the dissolution test solution. The experimental conditions were as follows, and the following dissolution tests of Experimental Examples 4 to 10 were also performed in the same manner as in Experimental Example 3.
[0073] · Eluate: pH1.2, 500ml
[0074] Dissolution samples: comparativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com