Hydrogen-containing polysiloxane photoinitiator terminated by photoinitiating group and preparation method thereof
A technology of hydrogen polysiloxane and photoinitiator, which is applied in the field of single-component polysiloxane photoinitiator, and can solve problems such as poor control of silicon hydrogen content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Preparation:
[0046] 1,1,3,3,5,5,7,7-octamethylcyclotetrasiloxane D 4 (1.2g, 4mmol), 1,3,5,7-tetramethylcyclotetrasiloxane D 4 H(0.05g, 0.21mmol), a mixture of anhydrous strongly acidic styrene-based cation exchange resin (Langfang Nanda Resin Co., Ltd.) (0.09g) and anhydrous toluene 5mL, after heating at 110°C for 5h, stop the reaction, and cool naturally After reaching room temperature, the reaction solution was filtered to remove the cation exchange resin, and low boilers were extracted under reduced pressure at 120°C to obtain 1.60 g of a yellow viscous liquid.
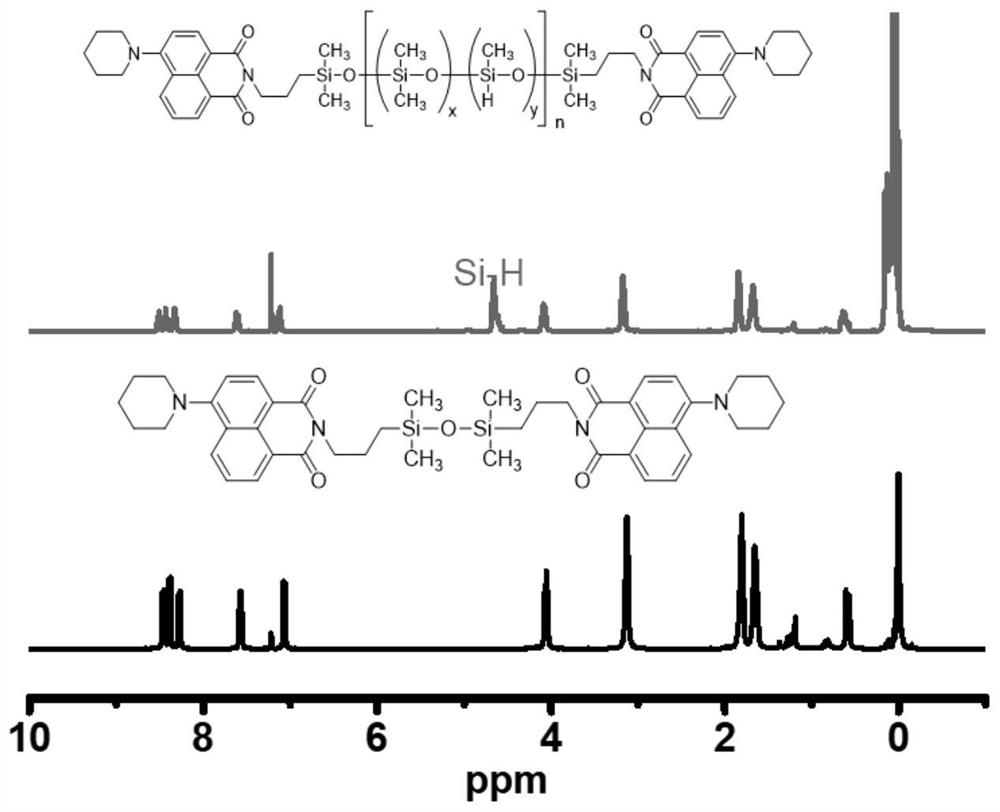
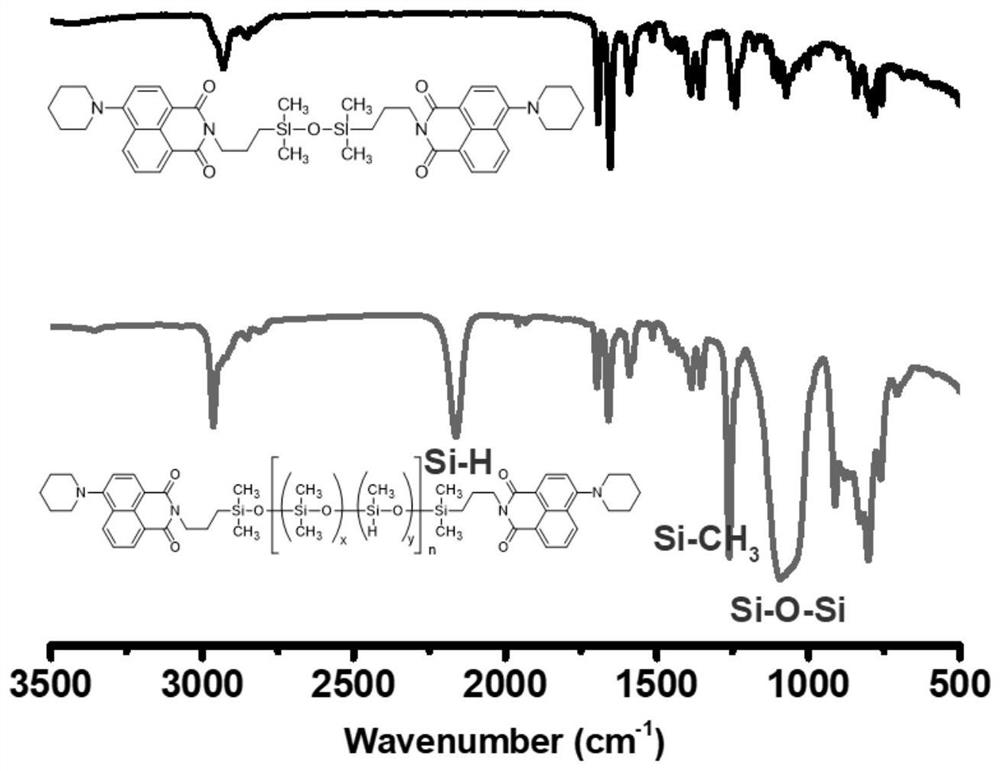
[0047] 1 H NMR (400MHz, CDCl 3 )δ(ppm)8.68–8.54(m,Ar-H),8.45-8.40(m,Ar-H),8.38-8.32(m,Ar-H),7.75–7.57(m,Ar-H),7.16 (t,Ar-H),4.69-4.55(m,Si-H),4.15-3.99(m,NCH 2 -),3.21(s,N(CH 2 CH 2 CH) 2 ),1.88(d,N(CH 2 CH 2 CH) 2 ),1.74-1.68(m,N(CH 2 CH 2 CH) 2 , Si-CH 2 CH 2 -),0.76–0.55(m, Si-CH 2 ),0.28–-0.04(m, Si-CH 3 );FT-IR(film,cm -1 ): ν=2162 (Si-H), 1697, 1659 (C=O), 1260 (Si-C...
Embodiment 2
[0051]
[0052] Preparation:
[0053] 1,3,5,7-Tetramethylcyclotetrasiloxane D 4 H (0.40g, 1.7mmol), a mixture of anhydrous strongly acidic styrene-based cation exchange resin (Langfang Nanda Resin Co., Ltd.) (0.2g) and 25mL of 1,2-dichloroethane, heated at 80°C for about 30h, Stop the reaction and cool down to room temperature naturally, filter the reaction solution to remove the cation exchange resin, and extract the low boilers under reduced pressure at 120°C to obtain 5.2 g of yellow viscous liquid.
[0054] 1 H NMR (400MHz, CDCl 3 )δ(ppm)8.68–8.54(m,Ar-H),8.45-8.40(m,Ar-H),8.38-8.32(m,Ar-H),7.75–7.57(m,Ar-H),7.16 (t,Ar-H),4.69-4.55(m,Si-H),4.15-3.99(m,NCH 2 -),3.21(s,N(CH 2 CH 2 CH) 2 ),1.88(d,N(CH 2 CH 2 CH) 2 ),1.74-1.68(m,N(CH 2 CH 2 CH) 2 , Si-CH 2 CH 2 -),0.76-0.55(m, Si-CH 2 ),0.28–-0.04(m, Si-CH 3 );FT-IR(film,cm -1 ): ν=2162 (Si-H), 1697, 1659 (C=O), 1260 (Si-CH 3 ), 1220-980 (Si-O-Si), 801 (Si-CH 3 ).
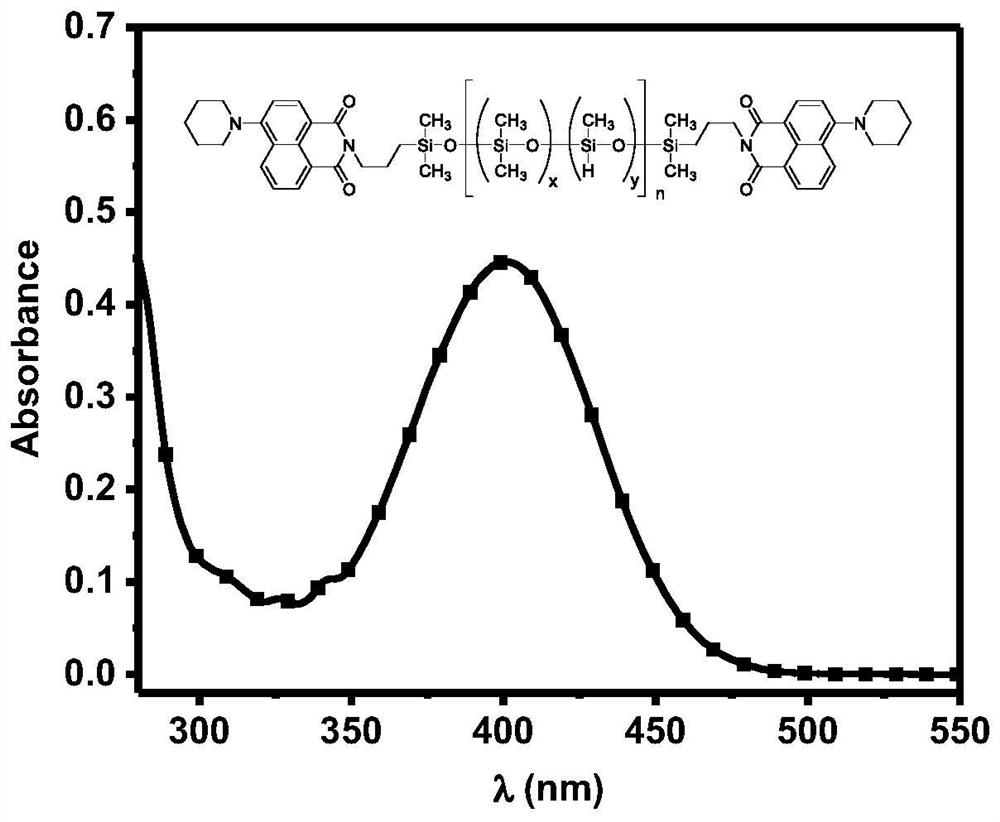
[0055] UV maximum absorption peak:...
Embodiment 3
[0058]
[0059] Preparation:
[0060] 1,3,5,7-Tetramethylcyclotetrasiloxane D 4 H (0.3g, 1.24mmol), a mixture of anhydrous strongly acidic styrene-based cation exchange resin (Langfang Nanda Resin Co., Ltd.) (0.06g) and anhydrous toluene 5mL, heated at 80°C for about 25h, then stopped the reaction and cooled naturally to At room temperature, the reaction solution was filtered to remove the cation exchange resin, and low boilers were extracted under reduced pressure at 140°C to obtain 0.75 g of a yellow viscous liquid.
[0061] 1 H NMR (400MHz, CDCl 3 )δ(ppm)8.68–8.54(m,Ar-H),8.45-8.40(m,Ar-H),8.38-8.32(m,Ar-H),7.75–7.57(m,Ar-H),7.16 (t,Ar-H),4.69-4.55(m,Si-H),4.15-3.99(m,NCH 2 -),3.21(s,N(CH 2 CH 2 CH) 2 ),1.88(d,N(CH 2 CH 2 CH) 2 ),1.74-1.68(m,N(CH 2 CH 2 CH) 2 , Si-CH 2 CH 2 -),0.76-0.55(m, Si-CH 2 ),0.28–-0.04(m, Si-CH 3 );FT-IR(film,cm -1 ): ν=2162 (Si-H), 1697, 1659 (C=O), 1260 (Si-CH 3 ), 1220-980 (Si-O-Si), 801 (Si-CH 3 ).
[0062] UV maximum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com