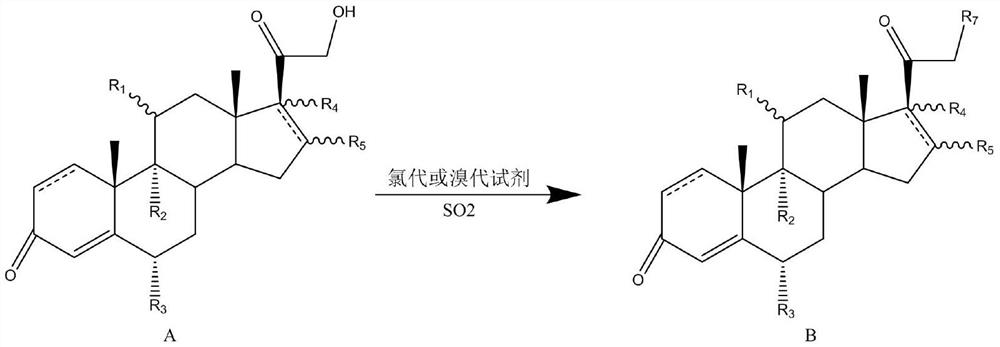
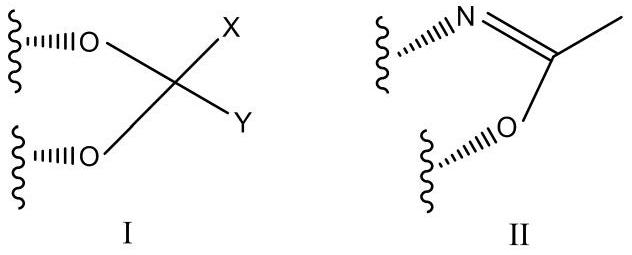
A kind of steroid 21 hydroxy chlorination or bromination method
A technology of bromination and chlorination, applied in the direction of organic chemical methods, chemical instruments and methods, steroids, etc., can solve the problems of expensive reagents, low reaction yield, and difficult removal of triphenylphosphine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] Compound 1 (5.0g) was dissolved in pyridine (30ml), nitrogen gas was introduced, the temperature was lowered to 0°C, chlorosuccinimide (2.5g) was added, and SO was added dropwise. 2 / pyridine (20% w / v to pyridine, 10 ml), after 1 h of reaction, the reaction solution was diluted into 500 ml of 0°C water, stirred for 1 h, filtered, and dried to obtain compound 2 (5.1 g, molar yield 97.2%) , purity 98.2% ).
Embodiment 2
[0052]
[0053] Compound 3 (1.0g) was dissolved in dimethylformamide (25ml), nitrogen was introduced, the temperature was lowered to 5°C, dichlorohydantoin (1.5g) was added, and SO was added dropwise. 2 / pyridine (20% by weight to pyridine, 4 ml), after 2 hours of reaction, the reaction solution was diluted into 100 ml of 0°C water, stirred for 1 hour, filtered, and dried to obtain compound 4 (1.0 g, yield 95.6%, purity 98.5% ).
Embodiment 3
[0055]
[0056]Compound 5 (15.0g) was dissolved in dichloromethane (500ml), the temperature was lowered to 10°C, chlorosuccinimide (10.0g) was added, lutidine (45ml) was added, and SO was passed through 2 (20% by weight to lutidine), after 0.5h of reaction, 100ml of water was added, and after stirring for 10min, the liquid was separated, and the organic phase was distilled under reduced pressure to remove the solvent to obtain compound 6 (15.3g, molar yield 98.2%, purity 9 8 . 9 % ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com