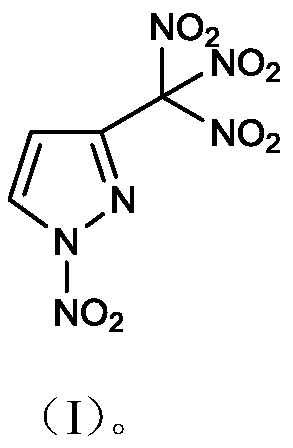
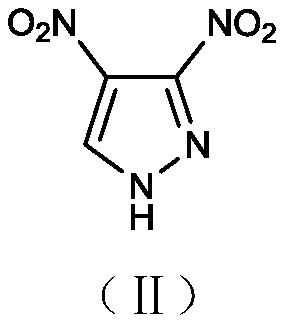
1-nitro-3-trinitromethylpyrazole compound
A technology of methylpyrazole and trinitro, which is applied in the field of pyrazole compounds and can solve the problems of low energy level and low oxygen balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
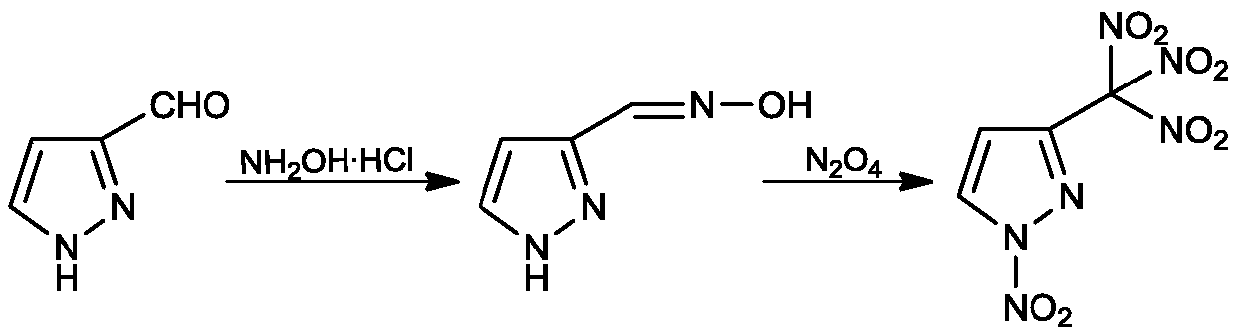
Embodiment 1
[0021] (1) Synthesis of 3-formaldehyde oxime pyrazole
[0022] Add 0.83g (12mmol) of hydroxylamine hydrochloride, 0.98g (12mmol) of sodium acetate and 40ml of ethanol into a 100ml three-necked flask, add 0.96g (10mmol) of 3-formaldehyde pyrazole under stirring, heat up to 60°C, and react for 2h. After the reaction, a milky white turbid liquid was obtained, cooled to room temperature, filtered, and the filtrate was evaporated to dryness to obtain an oily viscous solid, which was dissolved in 50ml of water and extracted three times with 50ml of ethyl acetate. The organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated. After drying, 0.93 g of a light yellow viscous solid was obtained, with a yield of 84.0%.
[0023] Structure Identification:
[0024] NMR spectrum: 1 H NMR (600MHz, dmso): δ11.65, 8.13, 7.65, 7.65, 7.63, 7.63, 7.54, 6.88, 6.88, 6.49, 6.49; 13 C NMR (151MHz, dmso): δ172.83, 170.89, 144.19, 142.62, 140.09, 138.70, 133.02, ...
Embodiment 2
[0040] (1) Synthesis of 3-formaldehyde oxime pyrazole
[0041] Add 1.25g (18mmol) of hydroxylamine hydrochloride, 1.47g (18mmol) of sodium acetate and 40ml of ethanol into a 100ml three-necked flask, add 0.96g (10mmol) of 3-formaldehydepyrazole under stirring, heat up to 50°C, and react for 2h. After the reaction, a milky white turbid liquid was obtained, cooled to room temperature, filtered, and the filtrate was evaporated to dryness to obtain an oily viscous solid, which was dissolved in 50ml of water and extracted three times with 50ml of ethyl acetate. The organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated. After drying, 0.96 g of light yellow viscous solid was obtained, and the yield was 86.8%.
[0042] Structure Identification:
[0043] NMR spectrum: 1 H NMR (600MHz, dmso): δ11.65, 8.13, 7.65, 7.65, 7.63, 7.63, 7.54, 6.88, 6.88, 6.49, 6.49; 13 C NMR (151MHz, dmso): δ172.83, 170.89, 144.19, 142.62, 140.09, 138.70, 133.02, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com