Mutant of human papilloma virus 35 L1 protein
A protein and variant technology, applied in the fields of molecular virology and immunology, can solve problems such as safety issues and increased production costs of HPV vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
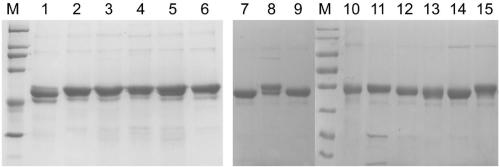
[0175] Example 1. Expression and purification of mutated HPV35 L1 protein
[0176] Construction of expression vector
[0177] A multi-point mutation PCR reaction is used to construct an expression vector encoding an HPV35 L1 protein containing a mutation derived from segment 1 of the HPV16 L1 protein, wherein the initial template used is the pTO-T7-HPV35 L1 plasmid (which encodes the HPV35L1 protein; It is abbreviated as 35L1 in Table 2). The templates and primers used in each PCR reaction are shown in Table 2, and the amplification conditions of the PCR reaction are set as follows: denaturation at 94°C for 10 minutes; 7 minutes 30 seconds); a final extension of 10 minutes at 72°C. The specific sequences of the PCR primers used are listed in Table 3.
[0178] Add 2 μL DpnI restriction endonuclease to the amplification product (50 μL), and incubate at 37° C. for 60 min. 10 μL of the digested product was used to transform 40 μL of competent Escherichia coli ER2566 prepared...
Embodiment 2
[0210] Example 2: Assembly of HPV virus-like particles and detection of particle morphology
[0211] Assembly of HPV virus-like particles
[0212] Take a certain volume (about 2ml) of protein H35-16T1, H35-16T2, H35-16T3, H35-16T4, H35-16T5, H35-16T1-31S2, H35-16T1-31S3, H35-16T1-31S4, H35-16T1 -31S5, H35-16T4-31S1, H35-16T4-31S2, H35-16T4-31S3, H35-16T4-31S5 were respectively dialyzed into (1) 2L storage buffer (20mM sodium phosphate buffer pH 6.5, 0.5M NaCl ); (2) 2L refolding buffer (50mM sodium phosphate buffer pH 6.0, 2mM CaCl 2 , 2mM MgCl 2 , 0.5M NaCl); and (3) 20 mM sodium phosphate buffer pH 7.0, 0.5M NaCl. Dialysis was performed for 12 h in each of the three buffers.
[0213] By similar method, HPV35 L1, HPV16N30 and HPV31 L1 proteins were assembled into HPV35 VLP, HPV16N30 VLP and HPV31 VLP, respectively.
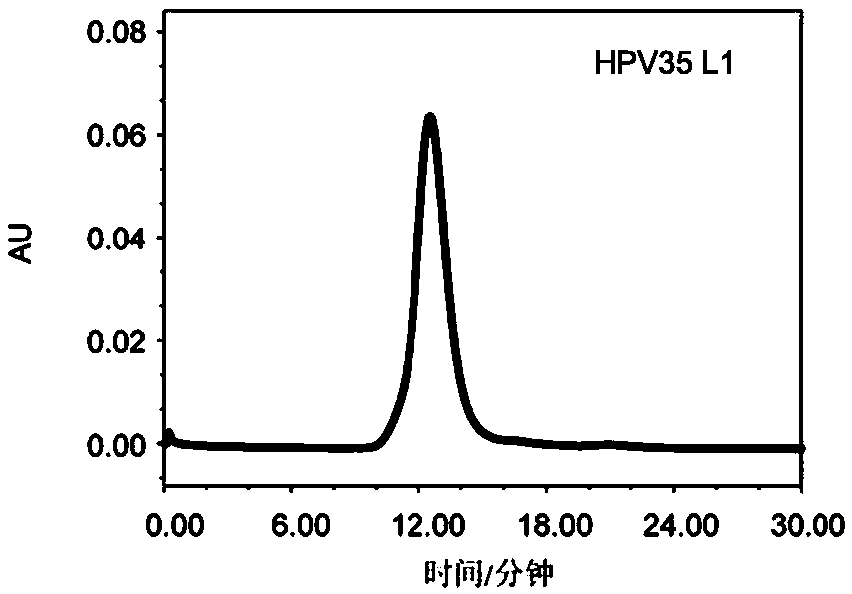
[0214] Molecular sieve chromatography analysis
[0215] A 1120 Compact LC high-performance liquid chromatography system from Agilent Corporation of the...
Embodiment 3
[0220] Example 3: Evaluation of thermal stability of virus-like particles
[0221]Use the differential temperature calorimeter VP Capillary DSC purchased from GE Company of the United States (formerly MicroCal Company) to evaluate H35-16T1, H35-16T2, H35-16T3, H35-16T4, H35-16T5, H35-16T1-31S2, H35- Thermal stability of VLPs formed by 16T1-31S3, H35-16T1-31S4, H35-16T1-31S5, H35-16T4-31S1, H35-16T4-31S2, H35-16T4-31S3, H35-16T4-31S5, wherein, The storage buffer of the protein was used as a control, and each protein was scanned from 10°C to 90°C at a heating rate of 1.5°C / min. Test results such as Figures 6A-6P shown. The results showed that the VLPs formed by each protein had extremely high thermal stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com