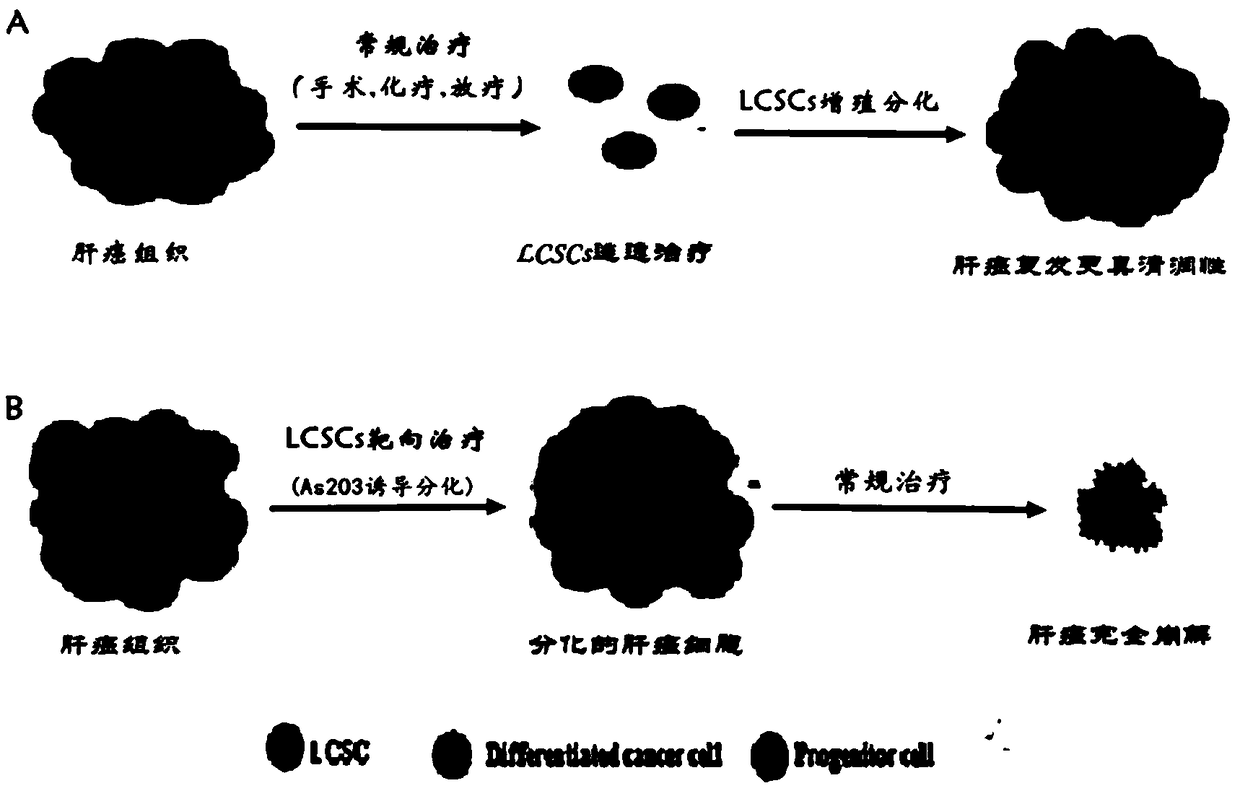
Pharmaceutical composition for removing residual liver cancer stem cells with combined use of arsenic trioxide and all-transretinoic acid and application thereof
An all-trans retinoic acid, liver cancer stem cell technology, applied in drug combinations, active ingredients of hydroxyl compounds, anti-tumor drugs, etc., can solve the problems of unsatisfactory anti-cancer effect, unclear treatment mechanism of HCC, and stagnation of anti-HCC research. Achieve the effect of removing liver cancer stem cells and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Arsenic reduces the expression of PML protein in LCSCs:
[0024] 【Purpose】:
[0025] Need to specify As 2 o 3 Whether to down-regulate the expression of PML protein in LCSCs.
[0026] 【experimental method】:
[0027] (1) CD133 + CD13 + LCSCs sorting: HuH7 cells were cultured in DMEM with 10% FBS at 37°C, 5% CO 2 , incubator with saturated humidity, and when the confluence of the cells reached 70-80%, the cells were digested with 0.25% trypsin to make a single-cell suspension, and the cells were counted, washed once with PBS, put into 1.5ml EP tubes, and set blank Control tube, CD13, CD133, CD13-CD133 double antibody four groups, add 5μl antibody to each tube, incubate at 4°C in the dark for 30min, centrifuge at 700r / min for 5min, discard supernatant, wash 3 times with PBS, resuspend cells in 300μl PBS, Flow cytometry (BD FACSAria II and BD FACSCalibur) was used to detect the expression of CD13 and CD133 markers and sort LCSCs.
[0028] (2) Immunofluorescence anal...
Embodiment 2
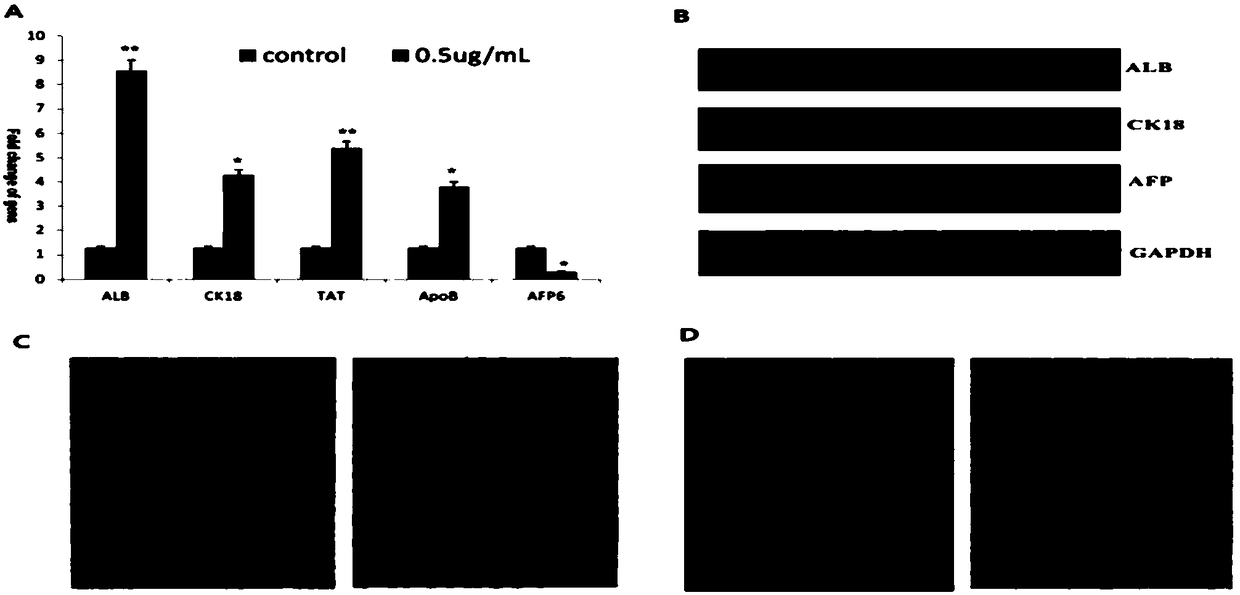
[0038] Arsenic induces LCSCS differentiation:
[0039] (1) Arsenic (As 2 o 3 ) Down-regulate the expression of Oct4, Sox2, Klf4, the key genes of liver cancer stem cells
[0040] 【Purpose】:
[0041] As has been found 2 o 3 Higher than 0.8μg / ml inhibits the proliferation of liver cancer cells, and may induce apoptosis of liver cancer cells at high concentrations. Now we need to observe 0.5μg / ml As 2 o 3 CD133 + CD13 + Whether the functional characteristics of LCSCs and the expression of key genes have any effect, and whether LCSCs can be induced to differentiate.
[0042] 【experimental method】:
[0043] ① CD133 + CD13 + LCSCs sorting: Liver cancer tissues and HuH7 cells were cultured in 10% FBS in DMEM, 37°C, 5% CO 2 , incubator with saturated humidity, and when the confluence of the cells reached 70-80%, the cells were digested with 0.25% trypsin to make a single-cell suspension, and the cells were counted, washed once with PBS, put into 1.5ml EP tubes respectively...
Embodiment 3
[0104] ATRA downregulates PML protein expression in LCSCs:
[0105] 【Purpose】:
[0106] Since ATRA can induce the differentiation of various solid tumor cells including cancer stem cells and breast cancer cells, it is necessary to determine what molecular pathways actually induce cell differentiation or inhibit the proliferation of liver cancer cells, and whether they have common target molecules with arsenic PML protein.
[0107] 【experimental method】:
[0108] (1) CD133 + CD13 + LCSCs sorting:
[0109] Liver cancer cells were cultured in DMEM with 10% FBS at 37°C and 5% CO 2 , incubator with saturated humidity, and when the confluence of the cells reached 70-80%, the cells were digested with 0.25% trypsin to make a single-cell suspension, and the cells were counted, washed once with PBS, put into 1.5ml EP tubes, and set blank Control tube, CD13, CD133, CD13-CD133 double antibody four groups, add 5μl antibody to each tube, incubate at 4°C in the dark for 30min, centrifu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com