Method for purifying antibody through cation exchange chromatography
A technology of cation exchange and chromatography, which is applied in the field of antibody purification, can solve the problems of low packing capacity, long operation time, and low packing utilization rate, and achieve low packing capacity, long operating time, and low packing utilization rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
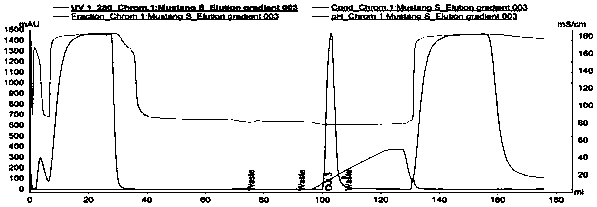
[0041] A cation chromatography membrane Mustang S XT Acrodis Units (PALL) was used with a membrane volume of 0.86 mL and an operating flow rate of 8.6 mL / min. The source of the sample is the monoclonal antibody (Mab1) expressed by CHO cells, which is captured by protein A affinity chromatography. After low pH inactivation, the pH of the sample antibody solution is adjusted to 5.5, and the conductivity value is 5mS / cm. The antibody solution is obtained after deep filtration in the middle . In the obtained antibody solution, the Mab1 concentration was 10.4 g / L, the purity was 97.0%, the multimer content was 2.5%, and the HCP content was 212.7 ppm.
[0042] After the chromatographic membrane was equilibrated with equilibration buffer (50mM NaAc-HAc, pH 5.5), 7.2mL diluted sample Mab1 concentration of 1.8g / L, purity of 97.0%, multimer content of 2.5% antibody solution was loaded. 15g / L MV. Use elution buffer (50mM NaAc-HAc, 0.5M NaCl, pH 5.5) for gradient elution (0-100%, 30MV),...
Embodiment 2
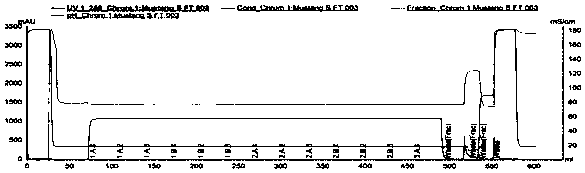
[0054] A cation chromatography membrane Mustang S XT Acrodis Units (PALL) was used with a membrane volume of 0.86 mL and an operating flow rate of 8.6 mL / min. The source of the sample is the monoclonal antibody (Mab1) expressed by CHO cells, which is captured by protein A affinity chromatography. After low pH inactivation, the pH of the sample antibody solution is adjusted to 5.5, and the conductivity value is 5mS / cm. The antibody solution is obtained after deep filtration in the middle . In the obtained antibody solution, the Mab1 concentration was 4.1 g / L, the purity was 97.0%, the multimer content was 2.5%, and the HCP content was 212.7 ppm.
[0055] Use equilibration buffer (50mM NaAc-HAc, pH 5.5, conductivity value 3.2mS / cm) to equilibrate the chromatographic membrane for 30MV. After loading 5.2mL of sample, the sample begins to break through and collect the flow-through. Continue to load the sample until 279mL is loaded, that is, when the antibody load is 1328g / LMV, the...
Embodiment 3
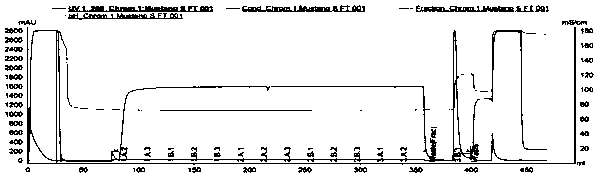
[0061] Prepacked column Fractogel COO-(Millipore), diameter 0.8cm, column height 10cm, column volume 5mL, operating linear flow rate 300cm / h. The source of the sample is the monoclonal antibody (Mab1) expressed by CHO cells, which is captured by protein A affinity chromatography. After low pH inactivation, the pH of the sample antibody solution is adjusted to 5.5, and the conductivity value is 5mS / cm. The antibody solution is obtained after deep filtration in the middle . In the obtained antibody solution, the Mab1 concentration was 10.4 g / L, the purity was 97.0%, the multimer content was 2.5%, and the HCP content was 212.7 ppm.
[0062] Equilibrate the chromatography column with equilibration buffer (50mM NaAc-HAc, pH 5.5) for 3CV, and after loading 32mL of the sample, the sample begins to break through, and the flow-through solution is collected. Continue to load the sample until 495mL is loaded, that is, when the antibody load is 1029g / L filler, the sample loading is compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com