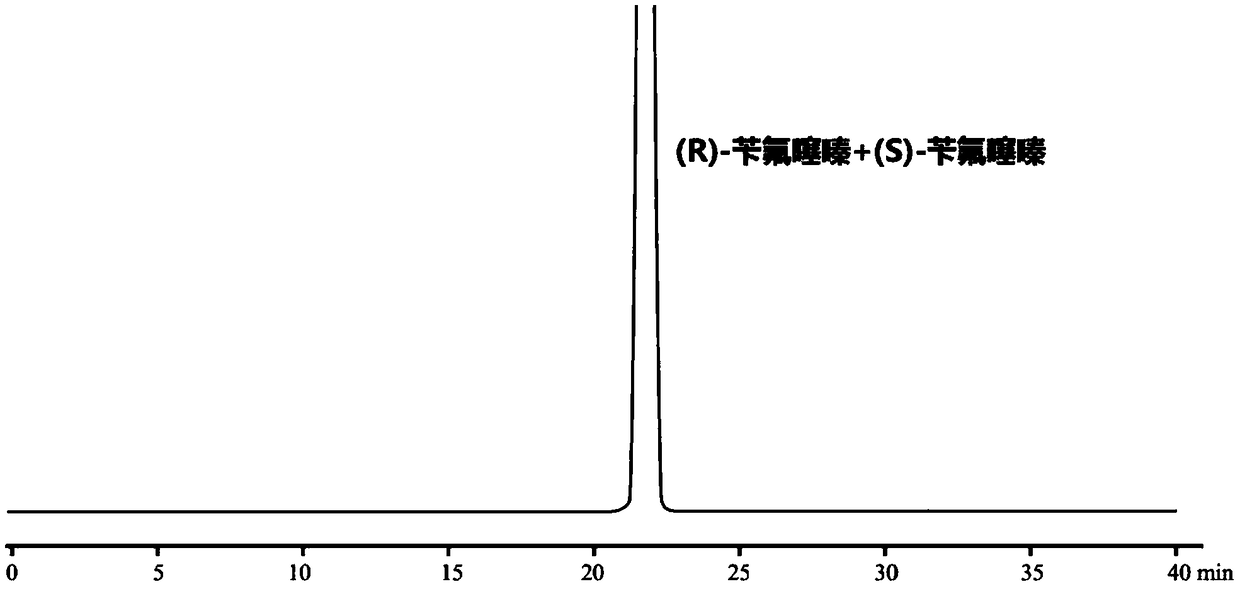
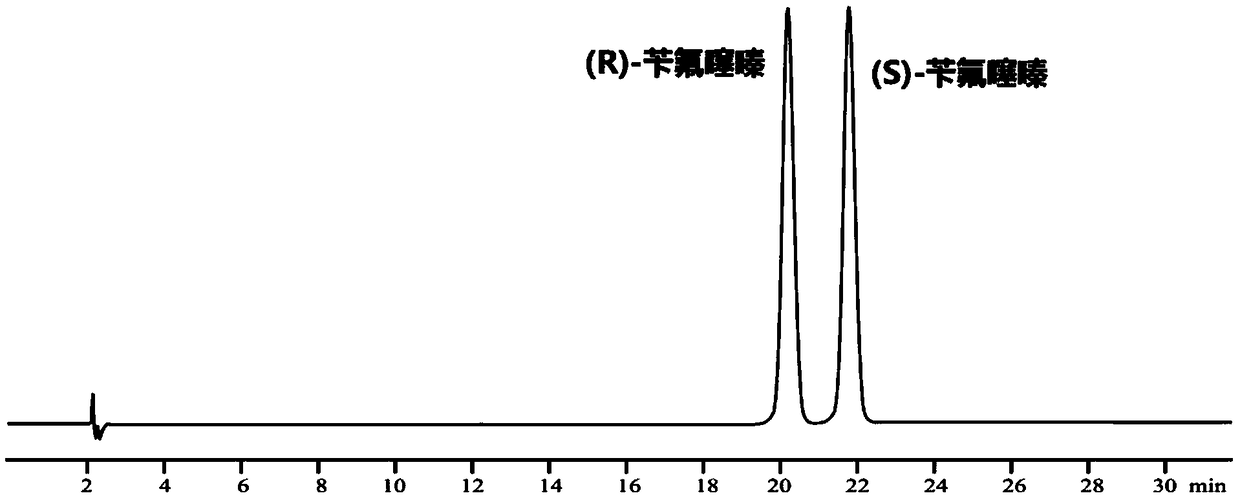
High performance liquid chromatography method for separating (R)-bendroflumethiazide and (S)-bendroflumethiazide
A technology of high performance liquid chromatography and bendroflumethiazide, which is applied in the field of chiral separation of diuretic drug bendroflumethiazide and methylchlorothiazide drug enantiomers, can solve the problem of high composition, achieve excellent separation effect and low detection cost cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 separates the HPLC method of (R)-bendroflumethiazide and (S)-bendroflumethiazide
[0065] 1. Experimental instruments and materials
[0066] Shimadzu LC-20AT high performance liquid chromatograph (equipped with LC-20AT pump, SIL-20A autosampler and SPD-M20A diode array detector, Japan Shimadzu company).
[0067] Waters Xbridge C18 chromatographic column (4.6mm×250mm, 5μm) is a product of Waters Company.
[0068] (R)-bendroflumethiazide and (S)-bendoflumethiazide standard substances were purchased or made by ourselves, with a purity of not less than 95%.
[0069] The chiral reagent (1R,2R)-2-aminocyclopentanecarboxylate was purchased with a purity of not less than 95%.
[0070] Acetonitrile and tetrahydrofuran were chromatographically pure, and water was purified water produced by Hangzhou Wahaha Group Co., Ltd.
[0071] 2. Experimental methods and results
[0072] 1. Solution preparation
[0073] Preparation of mobile phase A: water-acetonitrile-tetr...
Embodiment 2
[0089] Embodiment 2 separates the HPLC method of (R)-bendroflumethiazide and (S)-bendroflumethiazide
[0090] 1. Experimental instruments and materials
[0091] Shimadzu LC-20AT high performance liquid chromatograph (equipped with LC-20AT pump, SIL-20A autosampler and SPD-M20A diode array detector, Japan Shimadzu company).
[0092] Waters Xbridge C18 chromatographic column (4.6mm×250mm, 5μm) is a product of Waters Company.
[0093] (R)-bendroflumethiazide and (S)-bendoflumethiazide standard substances were purchased or made by ourselves, with a purity of not less than 95%.
[0094] The chiral reagent (1S,2R)-2-(Boc-amino) ethyl cyclopentanecarboxylate was purchased with a purity of not less than 95%.
[0095] Acetonitrile and tetrahydrofuran were chromatographically pure, and water was purified water produced by Hangzhou Wahaha Group Co., Ltd.
[0096] 2. Experimental methods and results
[0097] 1. Solution preparation
[0098] Preparation of mobile phase A: water-acet...
Embodiment 3
[0114] Embodiment 3 separates the HPLC method of (R)-methylchlorothiazide and (S)-methylchlorothiazide
[0115] 1. Experimental instruments and materials
[0116] Shimadzu LC-20AT high performance liquid chromatograph (equipped with LC-20AT pump, SIL-20A autosampler and SPD-M20A diode array detector, Japan Shimadzu company).
[0117] Waters Xbridge C18 chromatographic column (4.6mm×250mm, 5μm) is a product of Waters Company.
[0118] (R)-Methylchlorothiazide and (S)-Methylchlorothiazide standard products were purchased or self-made, with a purity of not less than 95%.
[0119] The chiral reagent (1R,2R)-2-aminocyclopentanecarboxylate was purchased with a purity of not less than 95%.
[0120] Acetonitrile and tetrahydrofuran were chromatographically pure, and water was purified water produced by Hangzhou Wahaha Group Co., Ltd.
[0121] 2. Experimental methods and results
[0122] 1. Solution preparation
[0123] Preparation of mobile phase A: water-acetonitrile-tetrahydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com