A method for synthesizing 3-methyloxetane-3-(4-nitrophenyl) carbonate
A technology of methyloxetane and nitrophenyl, which is applied in the field of scalable synthesis of 3-methyloxetane-3-yl carbonate, can solve the difficult separation of intermediates, yield, and Applicable to industrial scale-up production and other issues, to achieve a wide range of industrial application prospects and market value, easy operation, and the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
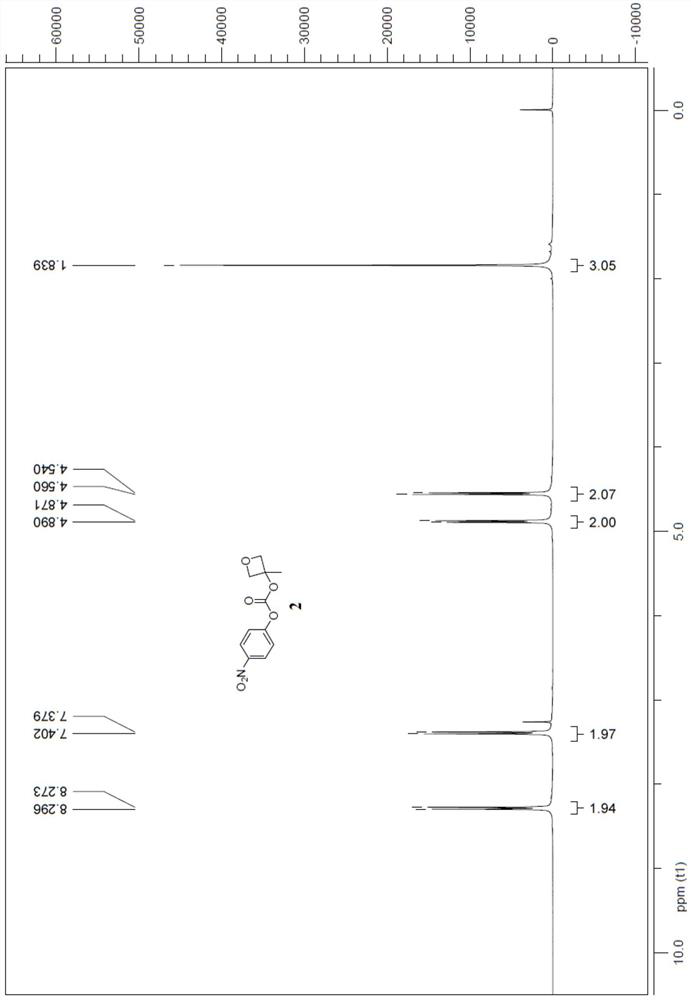
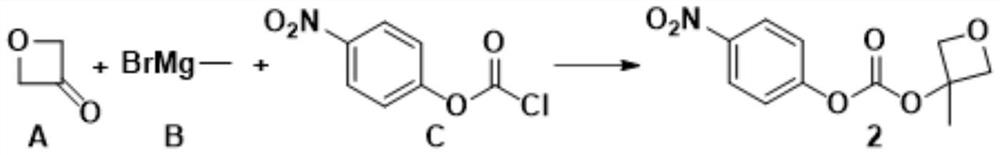
[0022] 3-Methyloxetan-3-yl (4-nitrophenyl) carbonate synthesis, the synthetic route is:
[0023]
[0024] The synthesis steps are:
[0025] Add 400g of anhydrous tetrahydrofuran and 200g of compound A into a dry 5L three-necked reaction flask, turn on mechanical stirring, pump air to replace the nitrogen three times, cool the reaction solution to -30°C, and keep it for half an hour, slowly add the compound A to the tetrahydrofuran solution Add 1.02L of methylmagnesium bromide B with a concentration of 3M dropwise, and keep the system temperature at -20°C. After the dropwise addition, keep the system temperature at -20°C for half an hour. Under the protection of nitrogen, slowly add 560g of compound C, and keep the system temperature At -15°C, keep the temperature of the system at -20°C and stir for 1 hour after the addition of the feed, spot the plate to detect the completion of the reaction, add saturated ammonium chloride to quench the reaction, extract with saturated sal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com