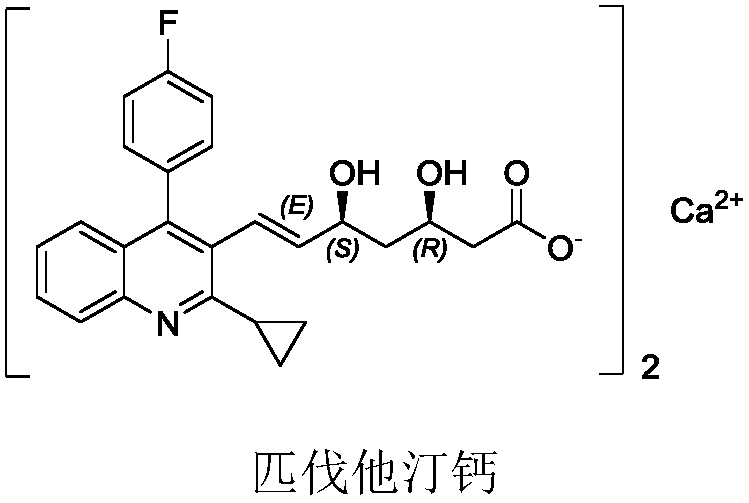
Pitavastatin calcium intermediate diastereomer and preparation method thereof
A technology of diastereomer, pitavastatin calcium, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
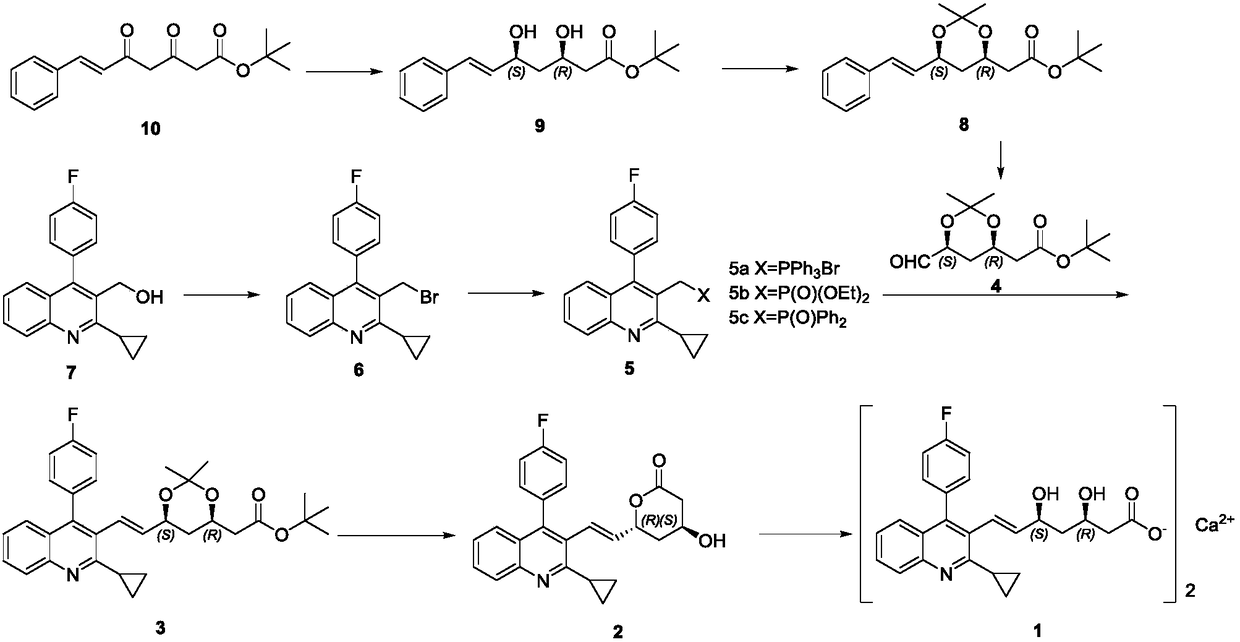
[0028] (5S,6E)-7-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3-oxo-5-hydroxy-6-heptenoic acid tert-butyl ester ( Compound Ⅲ) Preparation
[0029] Hexamethyldiazasilane (25.42g) was added to dry THF (100ml). -10℃ drop 2.5mol·L -1 The n-butyl lithium n-hexane solution (15.8 ml) was stirred at -10°C for 30 min. Cool to -30°C, add (S)-(-)-HYTRA (5.24g), and stir at -25°C for 30 min. After cooling to -78°C, 3-(2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl)prop-2-enal (5.0g) in THF (20ml) was added dropwise, Stirring was continued for 1 h at -78°C. Drop 2.5mol·L -1 N-Butyllithium n-hexane solution (31.5ml, 78.75mmol), continue stirring at -78°C for 30min, add tert-butyl acetate (10.16ml, 78.75mmol) dropwise, stir at -78°C for 1h, warm to 0°C and stir for 1h. The reaction was quenched by adding water (100ml), concentrated hydrochloric acid was adjusted to pH7, extracted with ethyl acetate (50ml×2), the organic layers were combined, the solvent was evaporated under reduced pressu...
Embodiment 2
[0031] (3S,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-6-heptenoic acid tert-butyl ester ( Preparation of compound Ⅱ)
[0032] Dissolve tetramethyltriacetoxyborazine (16.88g) and acetic acid (60ml) in acetonitrile (60ml) in sequence, stir at room temperature for 30min, cool to -15°C, and add compound III (6.10g) in acetonitrile (60ml) ) And acetic acid (20ml) solution, continue to stir for 3h. Saturated sodium bicarbonate solution was added to adjust pH 8, and methyl tert-butyl ether (200ml×2) was extracted. The organic layer was evaporated under reduced pressure to remove the solvent to obtain a pale yellow oily compound II (4.95g).
Embodiment 3
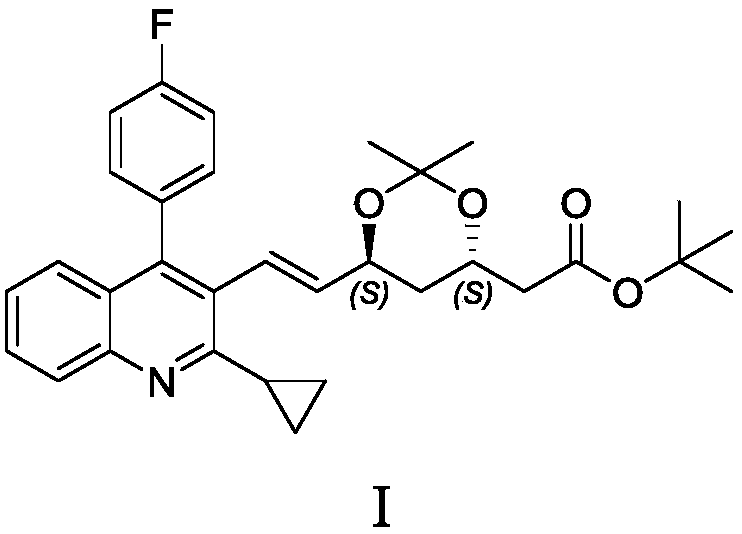
[0034] (3S,5S,6E)-7-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-3,5-O-isopropylidene Of tert-butyl 6-heptenoate (Compound Ⅰ)
[0035] (3S, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-6-heptenoic acid tert-butyl ester (4.5g) dissolved in 2,2-dimethoxypropane completely dissolved (in 45ml), added p-toluenesulfonic acid monohydrate (0.6g), stirred at room temperature for 2h, the reaction solution was evaporated to dryness under reduced pressure, and the residue was passed through silica gel Purification by column chromatography [eluent: n-hexane: ethyl acetate (10:1)] gave the target compound I (3.8 g) as a pale yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com