Thiourea derivative and thiohydracrylic acid parallel production method
A technology of thiourea derivatives and mercaptopropionic acid, which is applied in the direction of mercaptan preparation and organic chemistry, can solve the problems of restricting the industrial production or application of 3-mercaptopropionic acid, and the easy generation of hydrogen sulfide gas, etc., achieving short reaction time, The effect of mild reaction and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
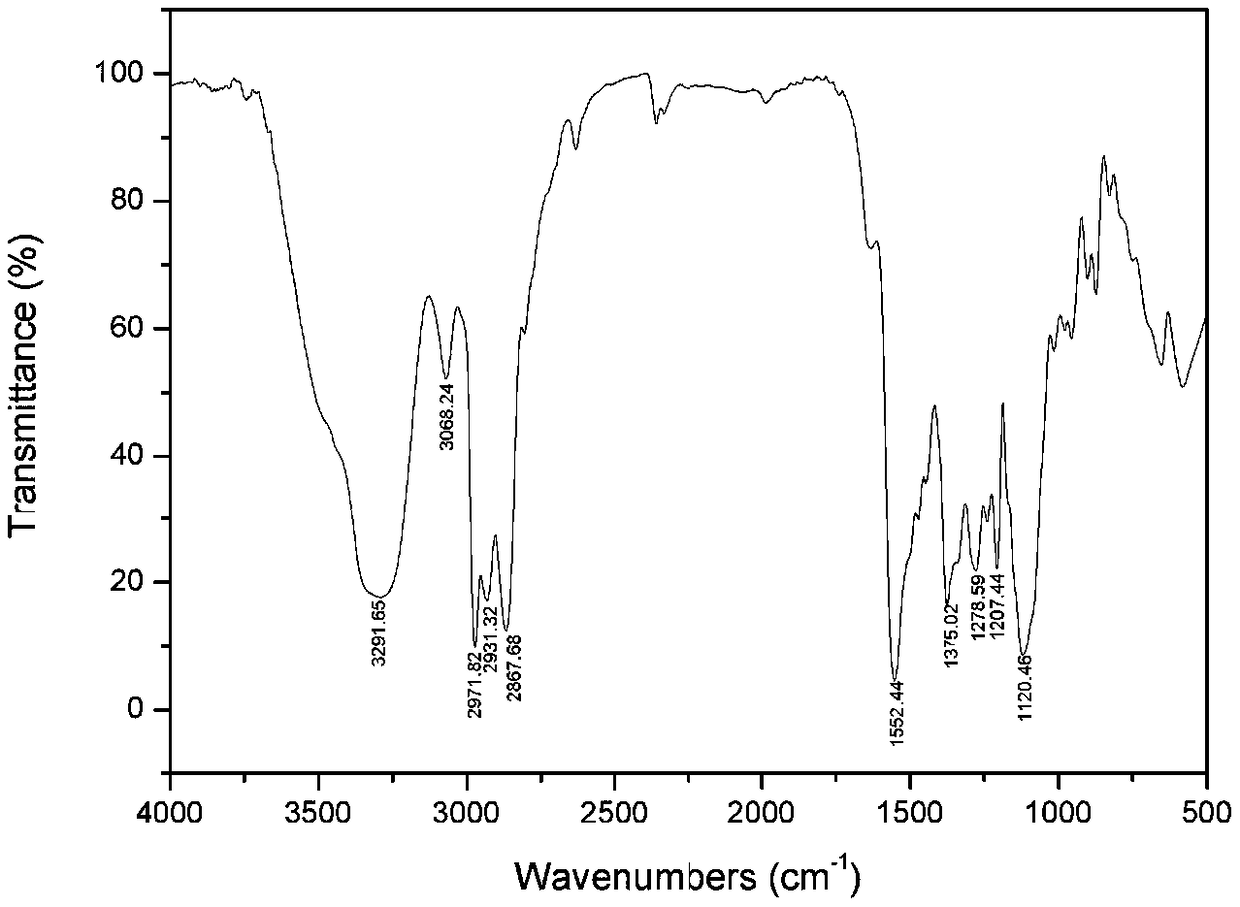
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of N-n-propyl-N'-isopropoxypropyl thiourea
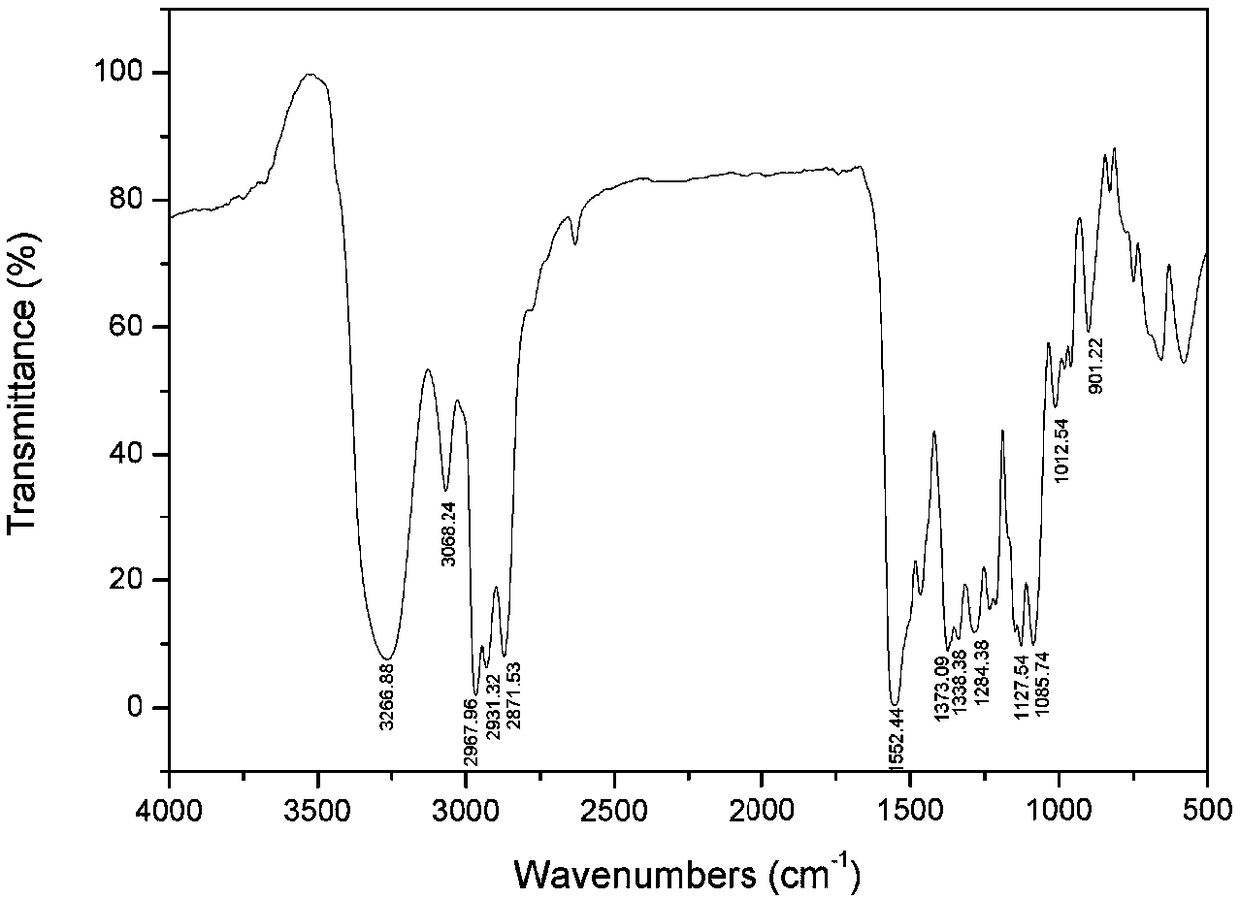
[0037] Add 21.13 parts of S-acrylic acid-N-n-propyl dithiocarbamate with a purity of 99% into the reactor, dissolve 4.07 parts of NaOH in 22.02 parts of water and add to the reactor, start stirring, and observe that the solid disappears Afterwards, the temperature was raised to 65° C., and then 13.14 parts of isopropoxypropylamine was added to react for 2 hours. After the reaction is over, add 19.82 parts of ethyl acetate into the reactor and stir it fully, let the upper layer of organic phase and the lower layer of water phase be separated, take the upper layer of organic phase and carry out vacuum distillation to obtain N-n-propyl-N'-iso Propoxypropylthiourea (infrared spectrum see figure 1 , see H-NMR diagram Image 6 , spectrogram data are shown in Table 1), and the yield was 95.6%. The pH of the lower aqueous phase was adjusted to 1-2 with HCl, then 19.82 parts of ethyl acetate was added for extr...
Embodiment 2
[0041] Example 2 N, the preparation of N'-di-n-propylthiourea
[0042] 6.44 parts of propylamine were used to replace 13.14 parts of isopropoxypropylamine, and the rest of the conditions were the same as in Example 1 to obtain a yield of 96.5% N, N'-di-n-propylthiourea and a yield of 93.6% of 3-mercaptopropionic acid.
Embodiment 3
[0043] Example 3 Preparation of N-isopropoxypropyl-N'-butoxypropylthiourea
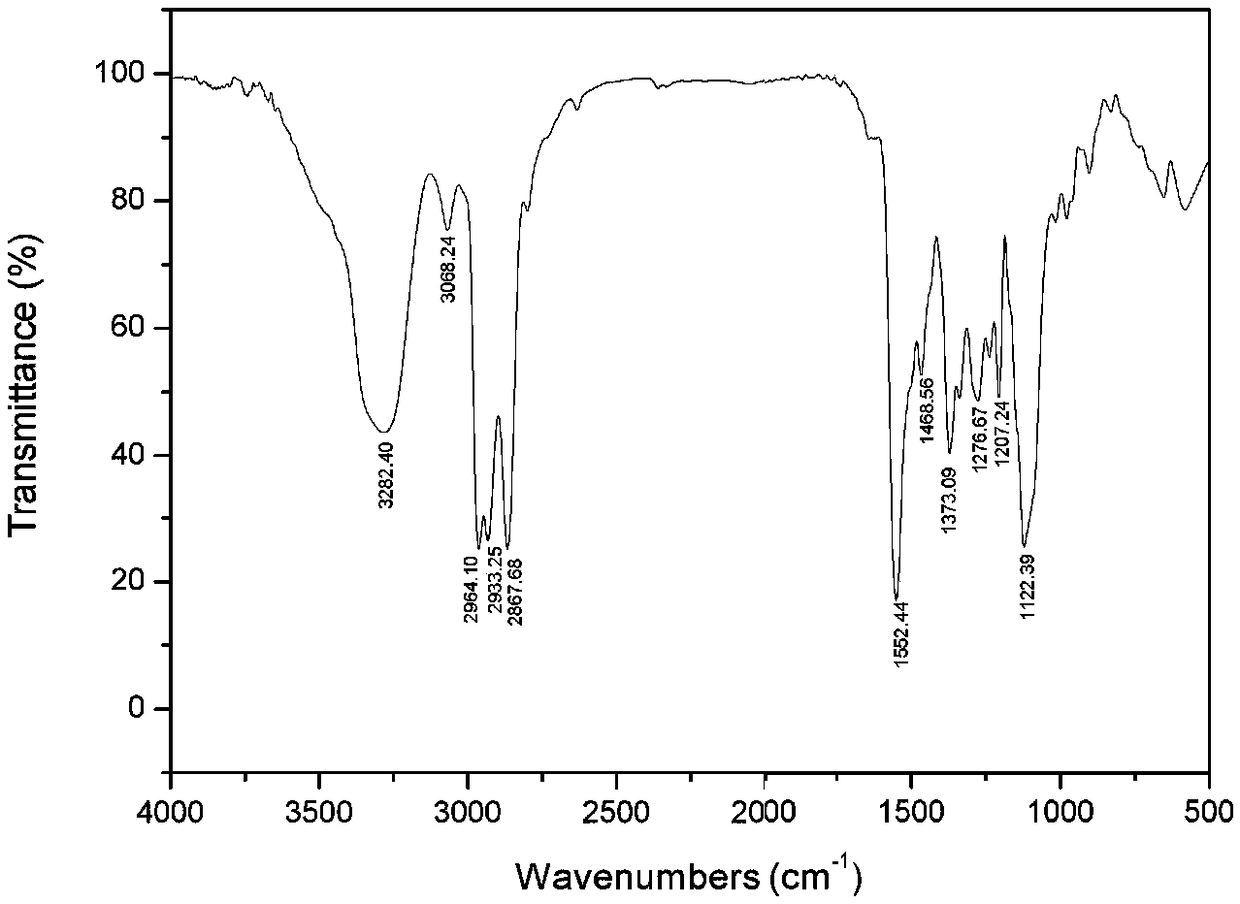
[0044] Add 25.18 parts of S-acrylic acid-N-isopropoxypropyl dithiocarbamate with a purity of 99% into the reactor, dissolve 3.79 parts of NaOH in 20.48 parts of water and add to the reactor, start stirring, and observe After the solution had no stratification, the temperature was raised to 70°C, and then 13.69 parts of butoxypropylamine was added and reacted for 2.5 hours. After the reaction is over, add 18.43 parts of ethyl acetate to the reactor and stir it fully, let it stand and separate the liquid to obtain the upper organic phase and the lower aqueous phase, take the upper organic phase for solvent removal, and obtain N-isopropoxypropyl-N '-butoxypropylthiourea (infrared spectrum see figure 2 ), the yield was 94.9%; the pH of the lower layer of the aqueous phase was adjusted to 1~2 with HCl, then 18.43 parts of ethyl acetate were added for extraction, liquid separation, the organic phase was t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap