A kind of method for preparing dodecanedioic acid
A technology of dodecanedioic acid and cyclododecanol, which is applied in the field of preparation of dodecanedioic acid, and can solve problems such as shortage, affecting product application performance, and mixed products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
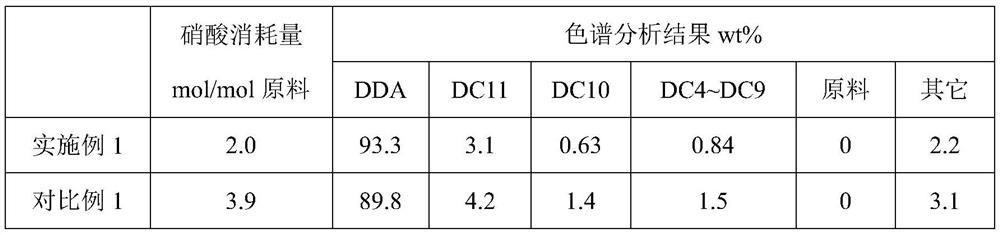
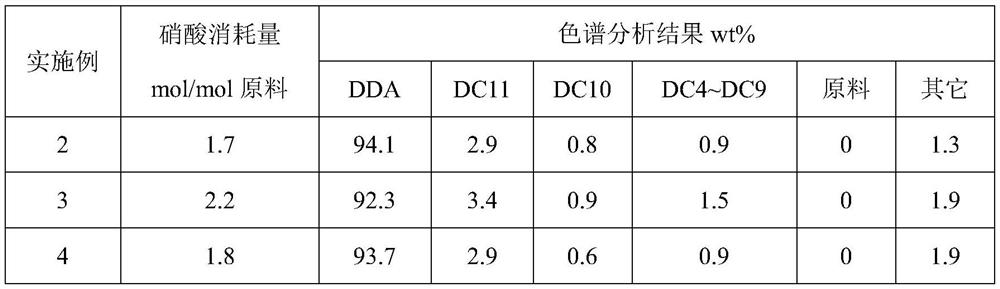
Embodiment 1
[0029] Add 266g of 65wt% nitric acid into a 500mL round bottom bottle, add copper nitrate and ammonium metavanadate so that the Cu and V contents are respectively 0.5wt% and 0.2wt%, and the system is heated to a reaction temperature of 75°C in an oil bath, and cyclododecanone / cyclododecanol is 95:5 by mass ratio and continues to join in the flask with molten state, keeps stirring state, keeps reaction temperature constant by controlling oil bath temperature, adds altogether 50g aforementioned raw materials (nitric acid / ( cyclododecanone) after 60min +cyclododecanol) molar ratio=10:1), crystals were precipitated, the reaction was stopped, and the average particle diameter D50=135m was read by an online particle size analyzer. The resulting slurry is quickly evaporated under high vacuum to remove volatile nitric acid and water, and the volatiles are replenished with a cold trap, and the captured nitric acid is weighed and analyzed for acidity, so as to determine the consumption...
Embodiment 2
[0036] Add 247g of 70wt% nitric acid into a 500mL round bottom bottle, add copper oxide and vanadium pentoxide so that the contents of Cu and V are respectively 0.5wt% and 0.2wt%, and the system is heated to a reaction temperature of 75°C through an oil bath, and cyclododecanone / cyclododecanol is 95:5 by mass ratio and continues to join in the flask with molten state, keeps stirring state, keeps reaction temperature constant by controlling oil bath temperature, adds altogether 50g aforementioned raw materials (nitric acid / ( cyclododecanone) after 45min +cyclododecanol) molar ratio=10:1), crystals were precipitated, the reaction was stopped, and the average particle diameter D50=228m was read by an online particle size analyzer. The resulting slurry is quickly evaporated under high vacuum to remove volatile nitric acid and water, and the volatiles are replenished with a cold trap, and the captured nitric acid is weighed and analyzed for acidity, so as to determine the consumpt...
Embodiment 3
[0038] Add 191g of 90wt% nitric acid into a 500mL round bottom bottle, add copper powder and ammonium metavanadate so that the contents of Cu and V are respectively 0.5wt% and 0.2wt%, and the system is heated to a reaction temperature of 65°C through an oil bath, and cyclododecanone / cyclododecanol is 95:5 by mass ratio and continues to join in the flask with molten state, keeps stirring state, keeps reaction temperature constant by controlling oil bath temperature, adds altogether 50g aforementioned raw materials (nitric acid / ( cyclododecanone) after 45min +cyclododecanol) molar ratio=10:1), a large amount of crystals were precipitated, the reaction was stopped, and the average particle diameter D50=298m was read by an online particle size analyzer. The resulting slurry is quickly evaporated under high vacuum to remove volatile nitric acid and water, and the volatiles are replenished with a cold trap, and the captured nitric acid is weighed and analyzed for acidity, so as to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com