Clean vanadium extracting method for high-calcium raw material containing vanadium
A high-calcium and raw material technology is applied in the field of vanadium extraction from high-calcium vanadium-containing raw materials and clean vanadium extraction from high-calcium vanadium-containing raw materials, which can solve problems such as complex processes and pollution, achieve efficient vanadium extraction, reduce evaporation, and reduce vanadium extraction. The effect of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
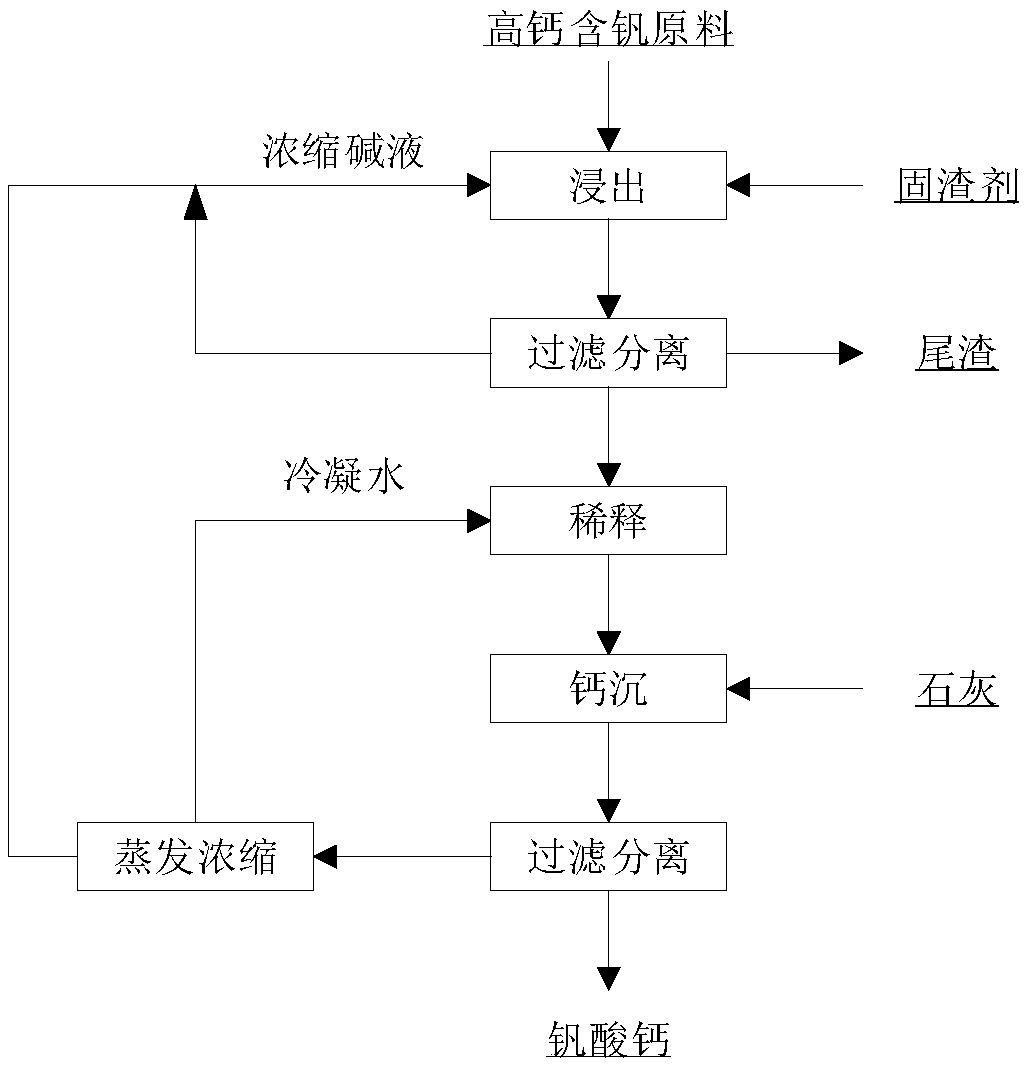
Image
Examples
Embodiment 1
[0061] Mix the concentrated lye, lime and steel slag with a CaO content of 30% evenly and add it to the pressure reaction kettle. 1% of vanadium-containing steel slag. The pressure leaching reaction temperature is 175°C, and the reaction time is 1h. After the reaction is completed, the reaction kettle is cooled to 70° C., the reaction kettle is opened, and the mixed slurry is filtered and separated to obtain a vanadium-containing leaching solution.
[0062] When the vanadium concentration in the leaching solution is 10g / L, dilute the leaching solution to a NaOH concentration of 10%, then add lime according to the Ca:V molar ratio of 0.4:1 for vanadium precipitation, the vanadium precipitation temperature is 90°C, and the vanadium precipitation time is 1h . The slurry after vanadium precipitation is filtered to obtain calcium vanadate and solution after vanadium precipitation. After vanadium precipitation, the solution is evaporated and concentrated to 20%, and then returned...
Embodiment 2
[0064] Mix the concentrated lye, lime and steel slag with a CaO content of 40% evenly and add it to the pressure reactor. 2% of vanadium-containing steel slag. The pressure leaching reaction temperature is 168°C, and the reaction time is 3h. After the reaction is completed, the reaction kettle is cooled to 90° C., the reaction kettle is opened, and the mixed slurry is filtered and separated to obtain a vanadium-containing leaching solution.
[0065] When the concentration of vanadium in the leaching solution is 12g / L, dilute the leaching solution to a NaOH concentration of 11%, and then add lime according to the ratio of Ca:V molar ratio of 0.6:1 for vanadium precipitation. The vanadium precipitation temperature is 92°C and the vanadium precipitation time is 1.5 h. The slurry after vanadium precipitation is filtered to obtain calcium vanadate and solution after vanadium precipitation. After the vanadium precipitation, the liquid is evaporated and concentrated to 22%, and th...
Embodiment 3
[0067] Mix the concentrated lye, calcium hydroxide and steel slag with a CaO content of 35% and add it into the pressure reactor. Calcium oxide is added in an amount of 3% of the vanadium-containing steel slag. The pressure leaching reaction temperature is 140°C, and the reaction time is 2h. After the reaction is completed, the reaction kettle is cooled to 85° C., the reaction kettle is opened, and the mixed slurry is filtered and separated to obtain a vanadium-containing leaching solution.
[0068] When the concentration of vanadium in the leaching solution is 13g / L, dilute the leaching solution to a NaOH concentration of 12%, then add lime according to the ratio of Ca:V molar ratio of 0.8:1 for vanadium precipitation, the vanadium precipitation temperature is 93°C, and the vanadium precipitation time is 2h . The slurry after vanadium precipitation is filtered to obtain calcium vanadate and solution after vanadium precipitation. After the vanadium precipitation, the liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com