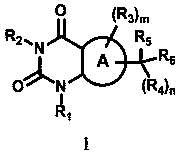
Pyrimidinedione derivative capable of inhibiting monocarboxylate transporter
A compound and solvate technology, applied in the field of cancer and autoimmune diseases, can solve problems such as rapid cell division cannot be maintained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0305] (S)-5-(4-Hydroxyisoxazolidine-2-carbonyl)-1-ethyl-3-methyl-6-(naphthalen-1-ylmethyl)-1,6-dihydro-2H -Pyrrolo[3,4-d]pyrimidine-2,4(3H)-dione (1).
[0306] .
[0307] Step A
[0308] 1-ethyl-3-methyl-6-(naphthalen-1-ylmethyl)-2,4-dioxo-2,3,4,6-tetrahydro-1 H -pyrrolo[3,4- d ] Pyrimidine-5-carbaldehyde (1a).
[0309] Intermediate A (210mg, 0.63mmol) was dissolved in anhydrous tetrahydrofuran (2.5ml), cooled to -78 degrees in a liquid nitrogen acetone bath, and then dropped into 2.5M n-butyllithium solution (378μl, 0.95mmol) to activate After 10 minutes, add 400 μl of anhydrous dimethylformamide, then slowly rise to room temperature, dilute with water, extract three times with ethyl acetate, combine the organic layers, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and separate by column chromatography (washing Removal of cyclohexane:ethyl acetate=10:1 to 8:1 and then 7:1) to obtain a yellow product (155.1 mg, yield 68%), namely 1-ethy...
Embodiment 2
[0321] ( S )-5-(4-Hydroxyisoxazolidine-2-carbonyl)-1-isobutyl-3-methyl-6-(naphthalen-1-ylmethyl)-1,6-dihydro-2 H -pyrrolo[3,4- d ]pyrimidine-2,4(3 H ) - diketone (2).
[0322] .
[0323] Example 2 is to prepare the title compound (2) according to the method of Example 1, replacing intermediate A in step A with intermediate B. MS-ESI (m / z): 477.0[M+H] + .
[0324] 1 H NMR (600MHz, CHLOROFORM-d) δ = 7.96 (br s, 1H), 7.92 - 7.88 (m, 1H), 7.87 - 7.84 (m, 1H), 7.58 - 7.50 (m, 2H), 7.44 (s, 1H), 7.19 - 7.13 (m, 1H), 6.25 (br s, 1H), 5.74 (br d, J=16.5 Hz, 2H), 4.77 - 4.64 (m, 1H), 4.57 -4.39 (m, 1H) , 4.24 - 4.12 (m, 1H), 4.08 - 3.95 (m, 1H), 3.64 - 3.43 (m, 3H), 3.37 (s, 3H), 2.14 - 2.05 (m, 1H), 0.94 - 0.78 (m, 6H).
Embodiment 3
[0326] ( S )-5-(4-Hydroxyisoxazolidine-2-carbonyl)-1-cyclopropylmethyl-3-methyl-6-(naphthalen-1-ylmethyl)-1,6-dihydro-2 H -pyrrolo[3,4- d ]pyrimidine-2,4(3 H )-diketones (3).
[0327] .
[0328] Example 3 is to prepare the title compound (3) according to the method of Example 1, replacing intermediate A in step A with intermediate C. MS-ESI (m / z): 475.0[M+H] + .
[0329] 1 H NMR (600MHz, CHLOROFORM-d) δ = 8.00 - 7.94 (m, 1H), 7.92 - 7.84 (m, 2H), 7.58 - 7.49 (m, 2H), , 7.48 - 7.41 (m, 1H), 7.20 ( br d, J=6.6 Hz,1H), 6.24 (br s, 1H), 5.71 - 5.62 (m, 1H), 5.00 - 4.89 (m, 1H), 4.72 (br s,1H), 4.53 - 4.45 (m , 1H), 4.19 (br d, J=7.7 Hz, 1H), 4.07 - 4.00 (m, 1H),3.82 - 3.68 (m, 2H), 3.52 (br s, 1H), 3.37 (s, 3H), 2.41 - 2.30 (m, 1H), 1.60 (br d, J=7.0 Hz, 1H), 1.28 - 1.21 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com