La/single-stranded binding protein (La/SSB) chimera antigen modified NK cell, and preparation method and application thereof
A technology of NK cells and host cells, applied in genetically modified cells, cells modified by introducing foreign genetic material, blood/immune system cells, etc. disorder, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
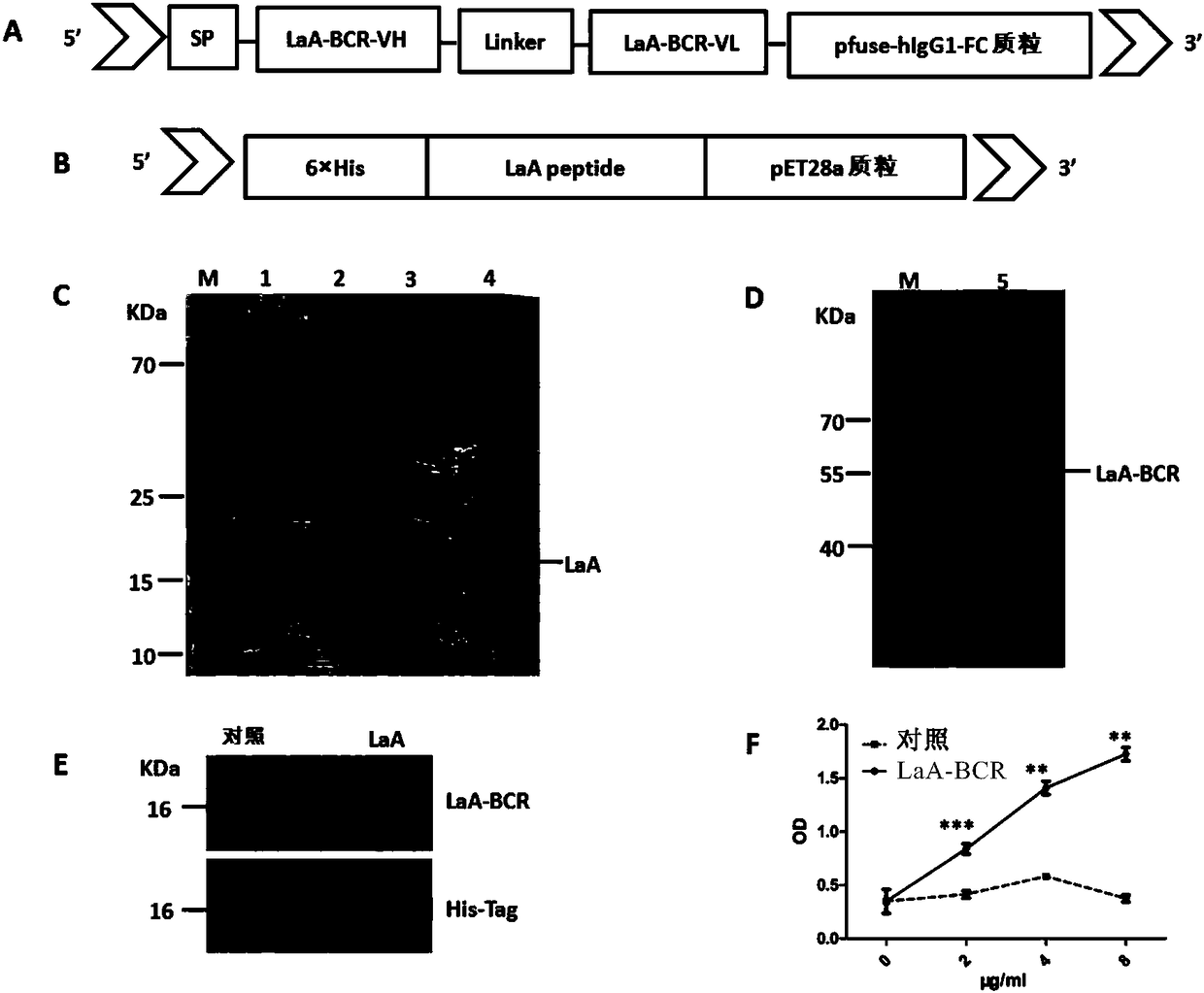
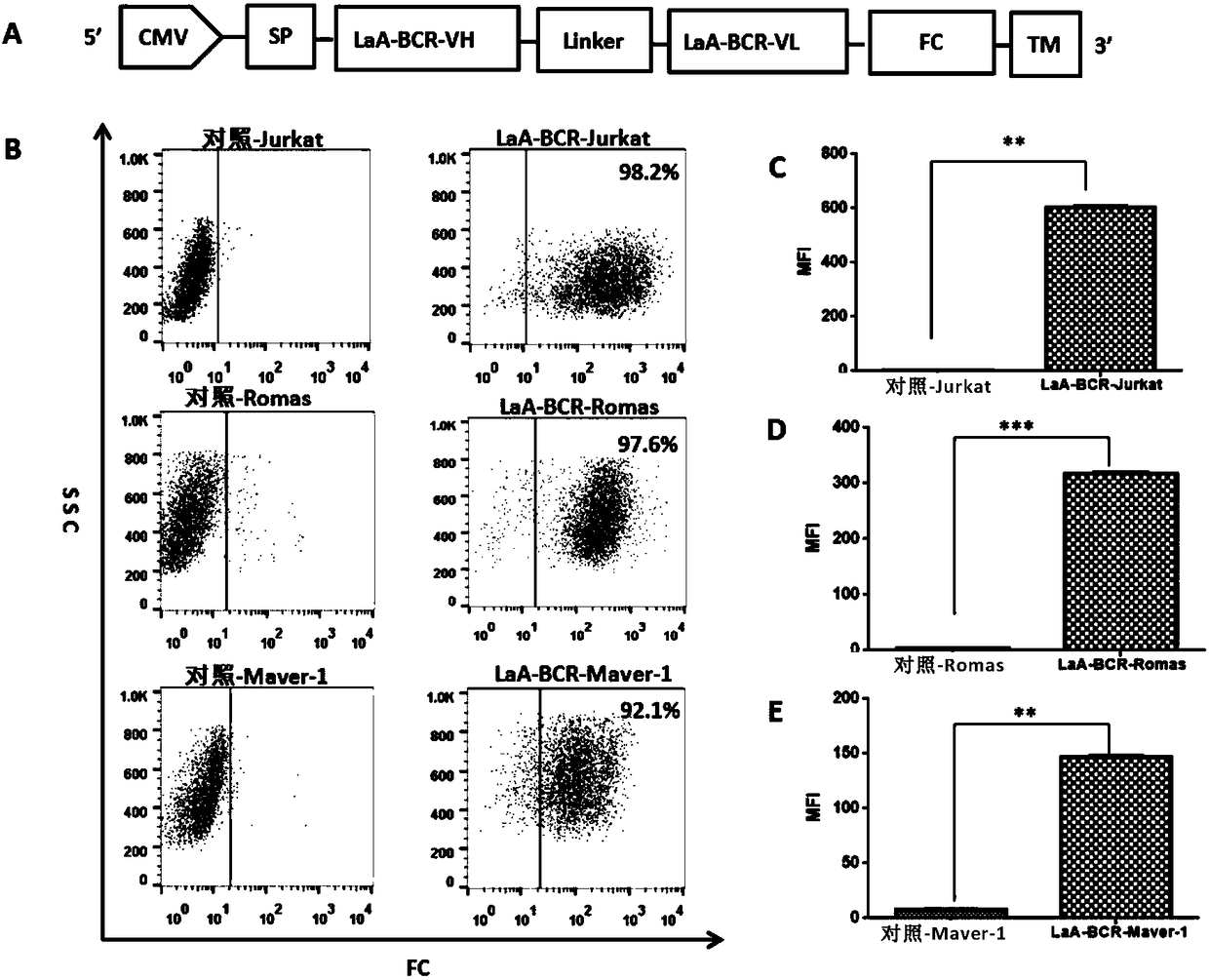
[0203] Embodiment 1 Purification of LaA protein and LaA-BCR protein
[0204] 1.1 Induction and purification of LaA protein
[0205] To induce the expression of soluble LaA protein, the cDNA encoding LaA protein was subcloned into pET28a prokaryotic expression vector (LaA-pET28a) containing 6×HIS ( figure 1 B), 6×HIS tag is used for protein purification. Then the LaA-pET28a plasmid was transformed into E.coli BL21(DE3) strain for culture.
[0206] The LaA protein was induced and purified according to the following steps: Pick a single LaA-pET28a clone and culture it overnight at 37°C in 3 ml LB medium containing 100 μg / mL kanamycin. Then continue to cultivate in the fresh culture medium that contains 100 μ g / mL kanamycin according to the ratio dilution of 1:100, when bacterium early logarithmic phase (OD=0.6-0.8), add 0.5mMisopropylβ-D-1-thiogalactopyranoside ( IPTG, Sangon Biotech) at 37°C for 2.5-3 hours. Then LaA protein was purified by Ni-NTA agarose (GE Healthcare Life...
Embodiment 2
[0211] Example 2 Affinity Determination of LaA-BCR Protein and LaA Antigen
[0212] Purified LaA antigen added to 5×SDS-PAGE loading buffer (P0015L, Beyotime, Shanghai, China) and non-LaA protein as a negative control were used as samples, separated by 12% SDS-PAGE, and the proteins on SDS-PAGE were separated by Transfer to PDVF membrane (IPVH00010, Millipore, MA), block with 5% skimmed milk at room temperature for 1 hour (Guangming, Shanghai, China), apply purified LaA-BCR protein and incubate overnight at 4°C, and then apply 0.1% Tween- After washing three times with 20 PBST, the PVDF membrane was incubated with horseradish peroxidase-conjugated anti-mouse IgG (H+L) secondary antibody (A10677, Life Technologies, CA). After washing three times with PBST, the PDVF membrane was visualized by chemiluminescence by applying the enhanced chemiluminescence kit (WBKLS0500, Millipore Corporation, MA).
[0213] Purified LaA protein was coated on ELISA microplate overnight at 4°C. The...
Embodiment 3
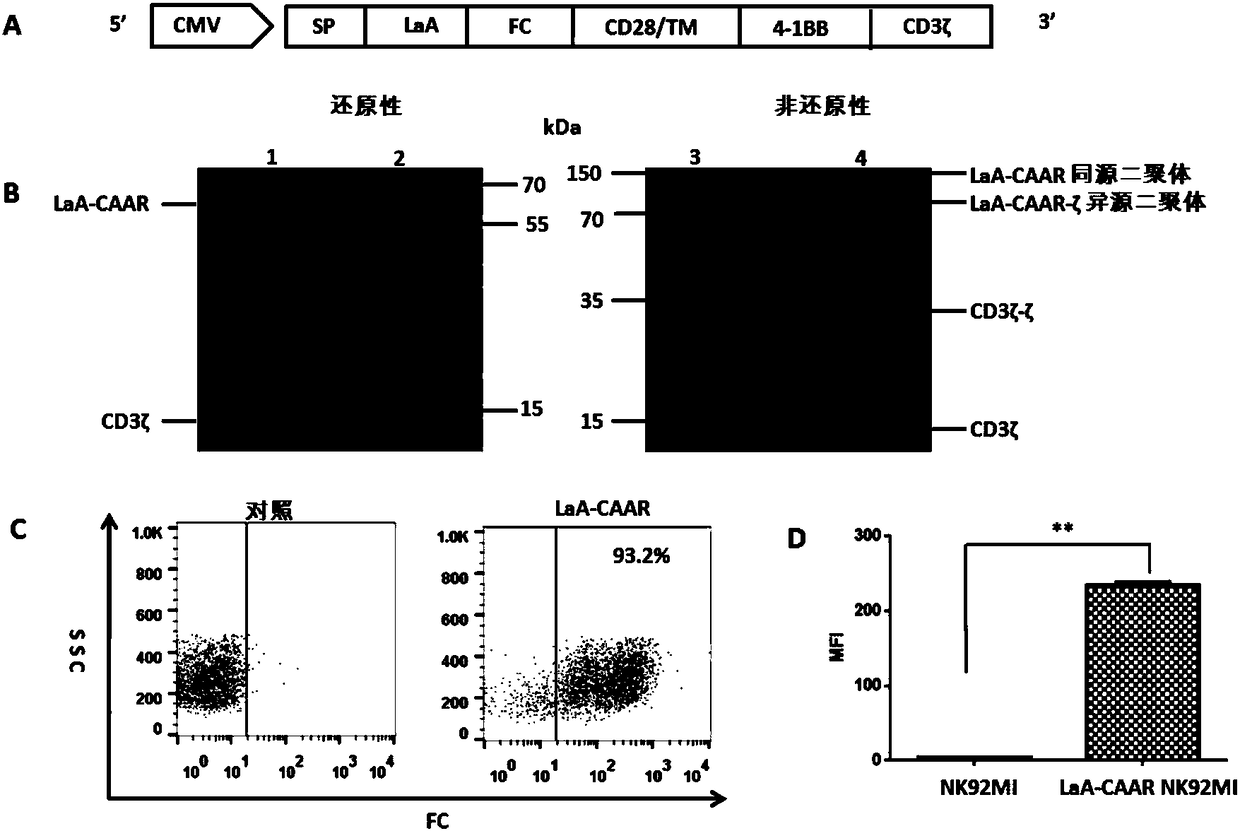
[0215] Example 3 Construction, characteristics and functional verification of LaA-CAAR NK92MI cells
[0216] 3.1 Structure design and lentiviral transfection of LaA-CAAR
[0217] The molecular structure design of LaA-CAAR includes: a CD8 transmembrane signal peptide guides the expression of LaA-CAAR on the surface of NK92MI cells, the LaA antigen fragment is used as an extracellular fragment, F C Linking fragments, CD28 molecule (comprising transmembrane region), 4-1BB co-stimulatory factor and CD3ζ signal region are connected to form a fusion protein ( figure 2 A). The LaA-CAAR fusion protein contains: leader sequence, LaA peptide, hinge domain (FC), CD28 transmembrane domain TM, two co-stimulatory domains (CD28 and 4-1BB) and CD3ζ intracellular signaling domain.
[0218] The SP-LaA-Fc-CD28 / TM-4-1BB-CD3ζ fusion gene sequence was gene-synthesized, and then constructed into the pCDH-CMV-MCS-EF1-CopPuro (System Biosciences) lentiviral expression vector. Plate 293T cells, pac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com