Combined inactivate vaccine for sheep pox and contagious caprine pleuropneumonia (CCPP), and production method of combined inactivate vaccine
A dual inactivated vaccine, pleuropneumonia technology, applied in biochemical equipment and methods, vaccines, multivalent vaccines, etc., can solve problems such as abortion of ewes, intradermal immunization, and death of lambs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
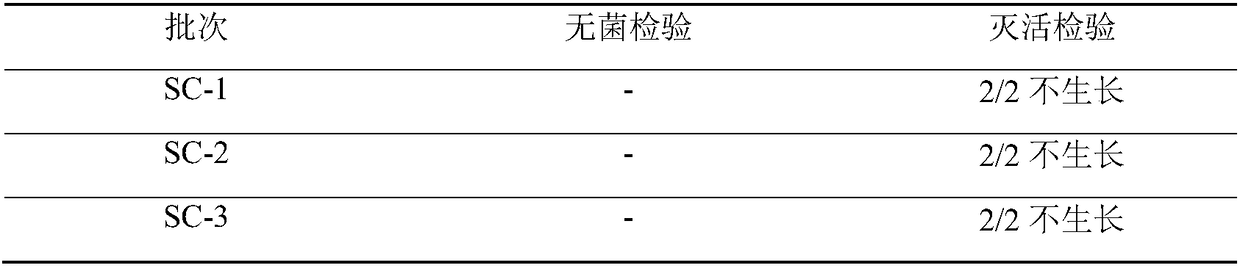
[0047] —— Vaccine preparation and testing
[0048] According to the established production process, three batches of inactivated vaccines for sheep pox and goat infectious pleuropneumonia were prepared, and the results are reported as follows.
[0049] 1. Materials
[0050] (1) The cells used for cell production are BHK-21 suspension cells.
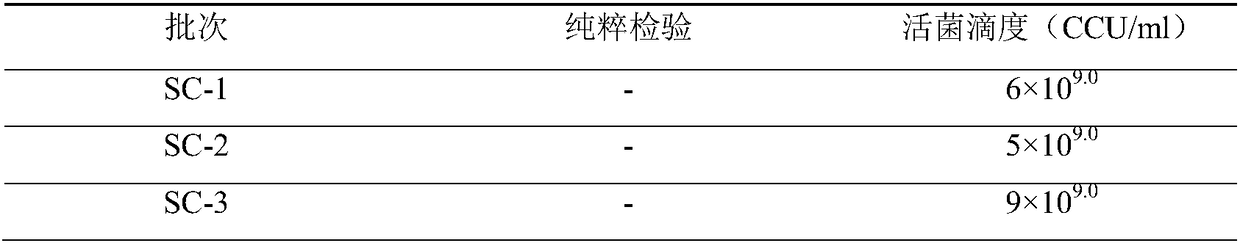
[0051] (2) Virus sheep pox virus AV41 strain and AV40 strain.
[0052] (3) Bacterial strains for strain production: goat infectious pleuropneumoniae Mycoplasma C87001 strain, F8 generation, live bacterial titer is 1×10 9.0 CCU / ml; Bacteria used for inspection: goat infectious pleuropneumonia C87002 strain tissue freeze-dried virus, code name 87002, generation: 52 generations, specification: 1.0g / bottle, identified, kept and supplied by the China Veterinary Drug Control Institute.
[0053] (3) Oil adjuvant Montanide ISA 201 adjuvant, produced by SEPPIC, France.
[0054] (4) The 3-month-old and 1-year-old experimental animals were healt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com