Synthetic method for temozolomide intermediate
A technology for the synthesis of temozolomide and its synthesis method, which is applied in the field of synthesis of temozolomide intermediates, can solve the problems of long steps and low yield, and achieve the effects of short preparation route, reduced production cost and increased safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
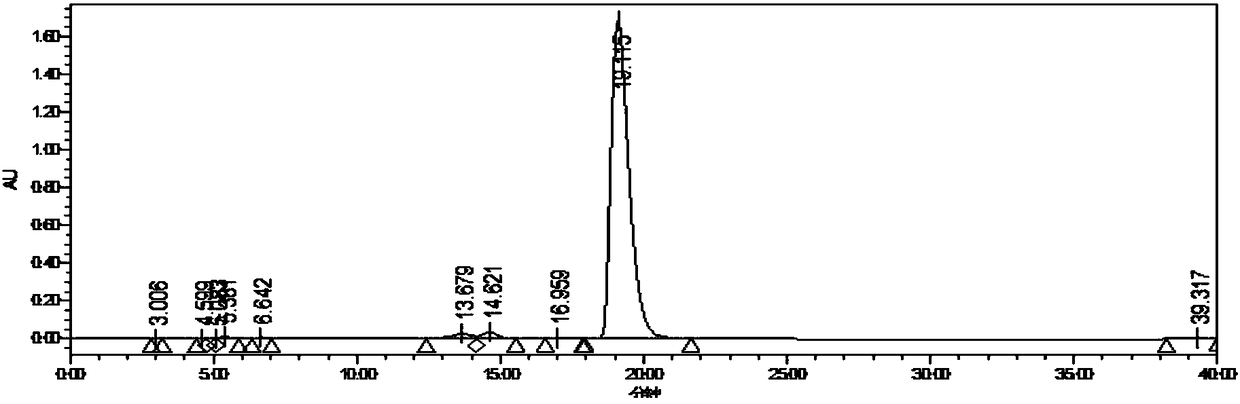
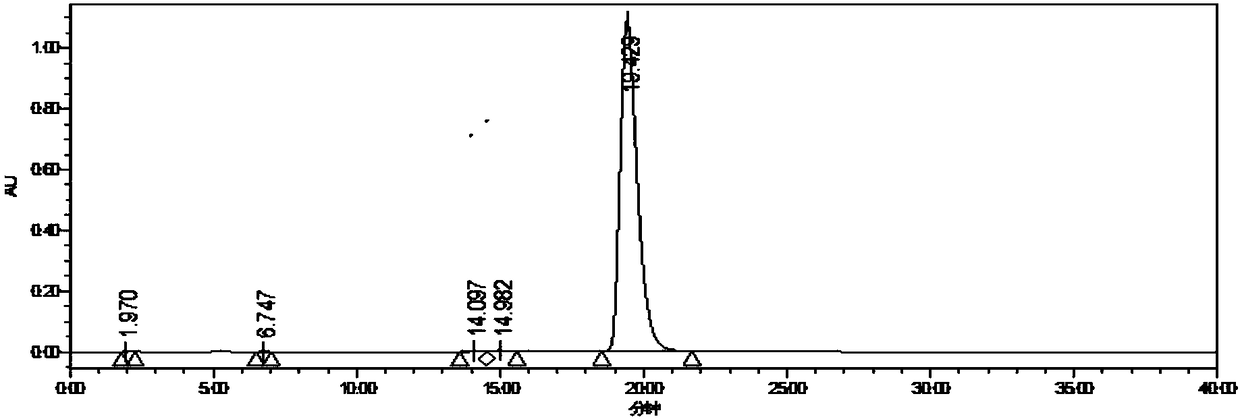
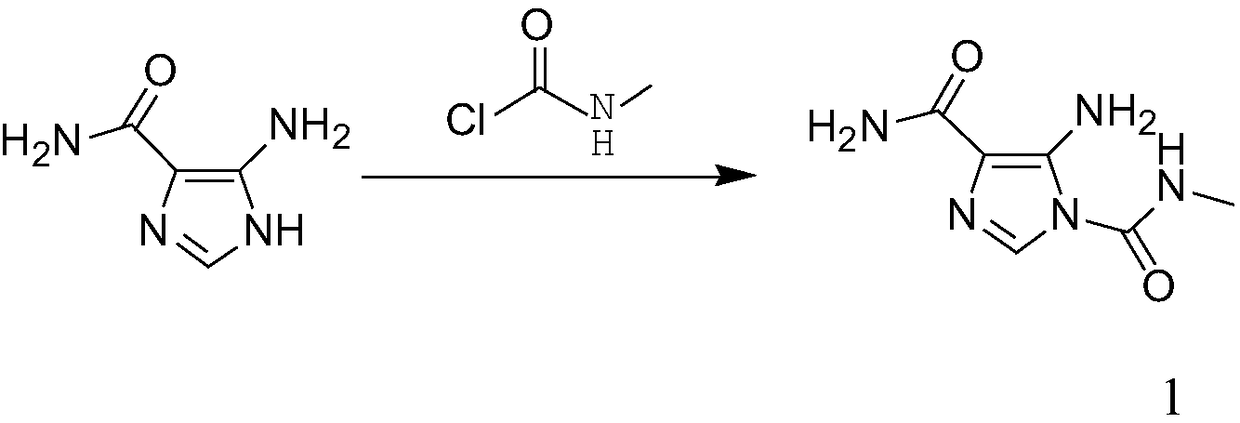
[0018] Add 5-aminoimidazole-4-carboxamide (160.0g, 1.27mol) and carbamoyl chloride (237.52g, 2.54mol) into acetonitrile (640mL), cool the solution to -5~5°C, add three Ethylamine (282.44mL, 2.03moL) was added at a rate of 10g / h, and the internal temperature was controlled at 0-10°C. After the addition, the temperature was raised to 10-20°C, and stirring was continued for 2h. HPLC monitoring showed that the raw material conversion rate was 95.95%, filtered, the filter cake was mixed with water (640mL) and stirred for 30min, filtered, the filter cake was washed with acetonitrile (48mL), and air-dried at 50°C for 12h to obtain Intermediate 1 (204.96g) , the yield was 89.60%, and the purity was 99.40%.
Embodiment 2
[0020] Add 5-aminoimidazole-4-carboxamide (160.0g, 1.27mol) and carbamoyl chloride (237.52g, 2.54mol) into acetonitrile (480mL), cool the solution to -5~5°C, add three Ethylamine (317.75mL, 2.29moL) was added at a rate of 10g / h, and the internal temperature was controlled at 10-20°C. After the addition, the temperature was raised to 10-20°C, and stirring was continued for 2h. HPLC monitoring showed that the conversion rate of the raw material was 87.10%, filtered, the filter cake was mixed with water (480mL) and stirred for 30min, filtered, the filter cake was washed with acetonitrile (48mL), and air-dried at 50°C for 12h to obtain Intermediate 1 (172.71g) , the yield was 75.50%, and the purity was 99.40%.
Embodiment 3
[0022] Add 5-aminoimidazole-4-carboxamide (160.0g, 1.27mol) and carbamoyl chloride (237.52g, 2.54mol) into acetonitrile (640mL), cool the solution to -5~5°C, add three Ethylamine (388.36mL, 2.79moL) was added at a rate of 10g / h, and the internal temperature was controlled at 20-30°C. After the addition, the temperature was raised to 10-20°C, and stirring was continued for 2h. HPLC monitoring showed that the conversion rate of the raw material was 90.43%, filtered, the filter cake was mixed with water (480mL) and stirred for 30min, filtered, the filter cake was washed with acetonitrile (54mL), and air-dried at 50°C for 12h to obtain Intermediate 1 (191.69g) , the yield was 83.80%, and the purity was 99.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com